Similar Questions
Explore conceptually related problems
Recommended Questions
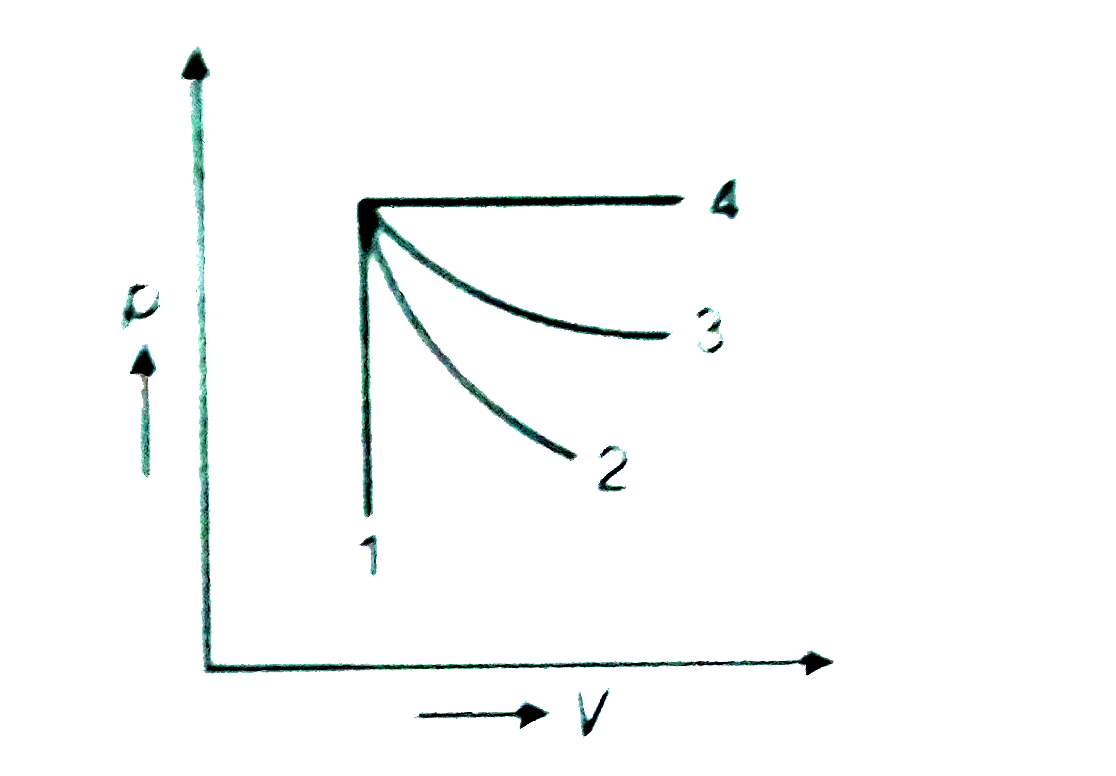
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- An ideal gas undergoes four different processes from the same initial ...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- The given diagram shows four processes i.e., isochoric, isobaric, isot...
Text Solution
|
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- What are isobaric, isochoric, isothermal and adiabatic processes?
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|