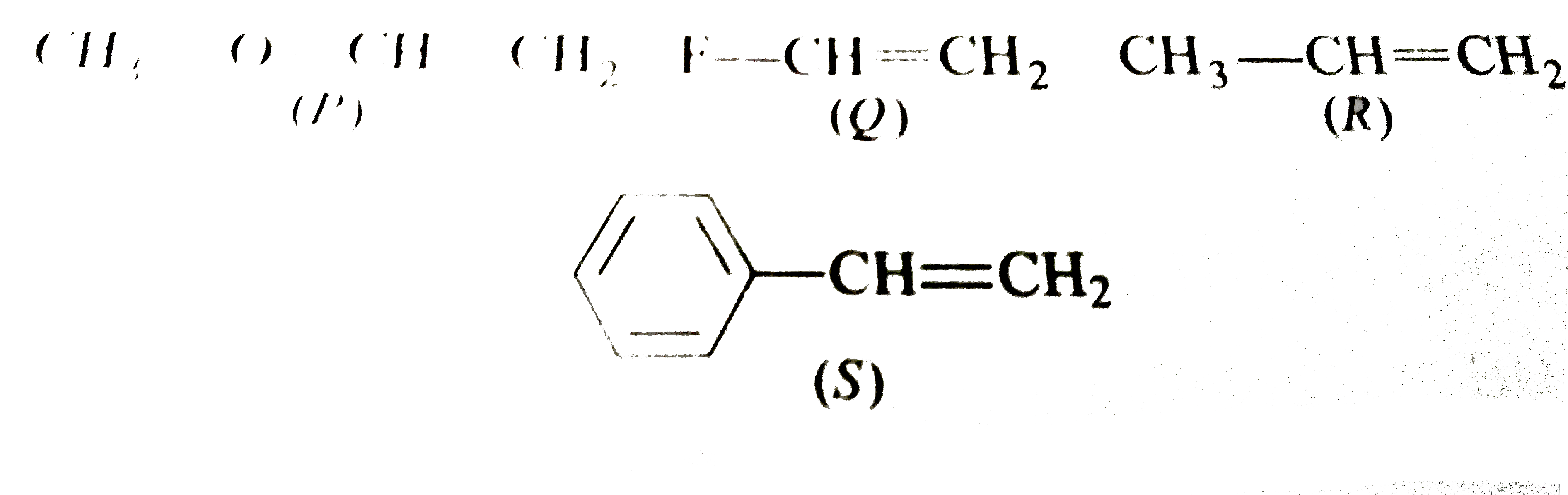Similar Questions
Explore conceptually related problems
Recommended Questions
- Rates of hydration of the following alkene are: CH(3)-OCH=CH(2)" "F...
Text Solution
|
- Name of the following as substituted derivatives of ethylene. (a) C...
Text Solution
|
- Which of the following is an alkene? (i) CH(3) - CH = CH(2) (ii) CH(2)...
Text Solution
|
- Write the IUPAc name of the following compound : (a) CH(3)-CH(2)-ov...
Text Solution
|
- The following two isomeric alkenes are related as- (I) CH(3)-CH=CH-C...
Text Solution
|
- IUPAC name is CH(3)-CH=CH-CH=CH(2)
Text Solution
|
- Rates of hydration of the following alkene are: CH(3)-OCH=CH(2)" "F...
Text Solution
|
- The IUPAC name for CH(3)CH(2)CH (CH = CH(2)) CH(2)CH(2)CH(3) is
Text Solution
|
- The IUPAC name of {:(CH(2) = CH-CH-CH(2)-CH(3)),(" |"),(" ...
Text Solution
|