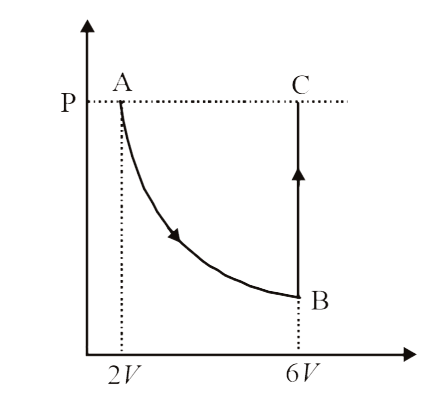
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-THERMODYNAMICS-Physics
- One mole of an ideal gas at an initial temperature true of TK does 6R ...
Text Solution
|
- For adiabatic process of an ideal gas the value of (dP)/P is equals to
Text Solution
|
- If 300 ml of a gas at 27^(@) is cooled to 7^(@) at constant pressure, ...
Text Solution
|
- A sample of gas expands from volume V(1) to V(2). The amount of work d...
Text Solution
|
- How much work to be done in decreasing the volume of an ideal gas by a...
Text Solution
|
- One mole of a perfect gas in a cylinder fitted with a piston has a pre...
Text Solution
|
- Work done by 0.1 mole of a gas at 27^(@)C to double its volume at cons...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- When heat is given to a gas in an isothermal change, the result will b...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- An ideal gas expands in such a manner that its pressure and volume can...
Text Solution
|
- In the following P-V diagram two adiabatics cut two isothermals at tem...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure,
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- Assertion: The isothermal curves intersect each other at a certain poi...
Text Solution
|
- Assertion: In adiabatic compression, the internal energy and temperatu...
Text Solution
|
- Statement I: The specific heat of a gas in an adiabatic process is zw...
Text Solution
|