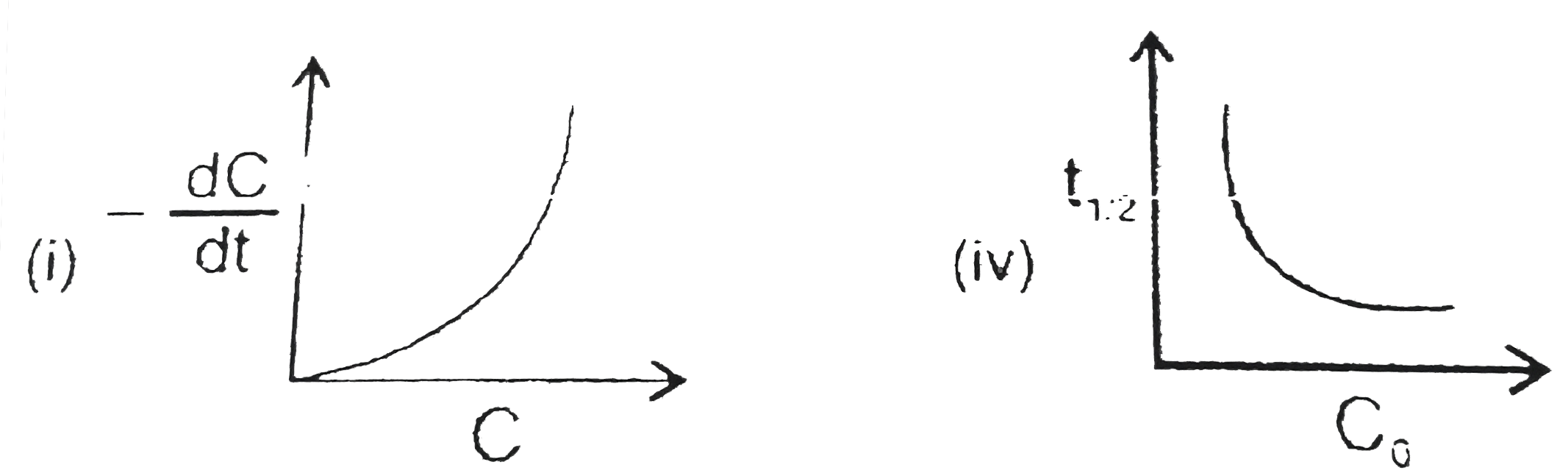A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
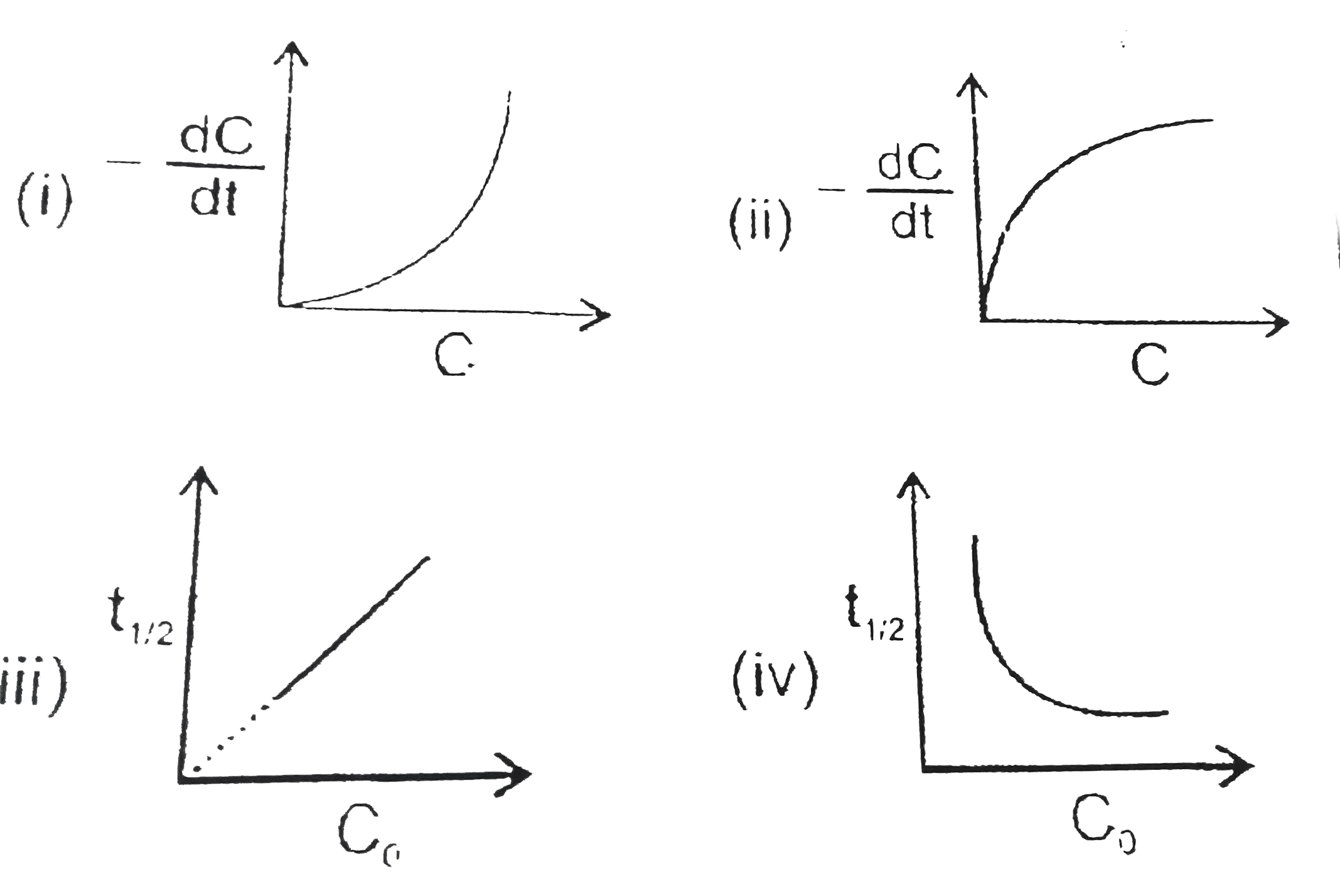
- Which of the following graphs are correct for a second order reaction:...
Text Solution
|
- Using velocity time graph, establish the relation s = ut + (1)/(2)at^(...
Text Solution
|
- Use velocity time graph to derive the relation : v^(2)-u^(2)=2 as, whe...
Text Solution
|
- The correct relation for impact parameter, where symbols have their us...
Text Solution
|
- Which of the following graphs are correct for a second order reaction:...
Text Solution
|
- Which of the following graphs is incorrect regarding rate constant (k)...
Text Solution
|
- Which of the following graphs is correct for second order reaction ?
Text Solution
|
- In following symbols are having usual meaning then which of the follow...
Text Solution
|
- Which of the following is correct ? (symbols have their ususal meaning...
Text Solution
|