A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
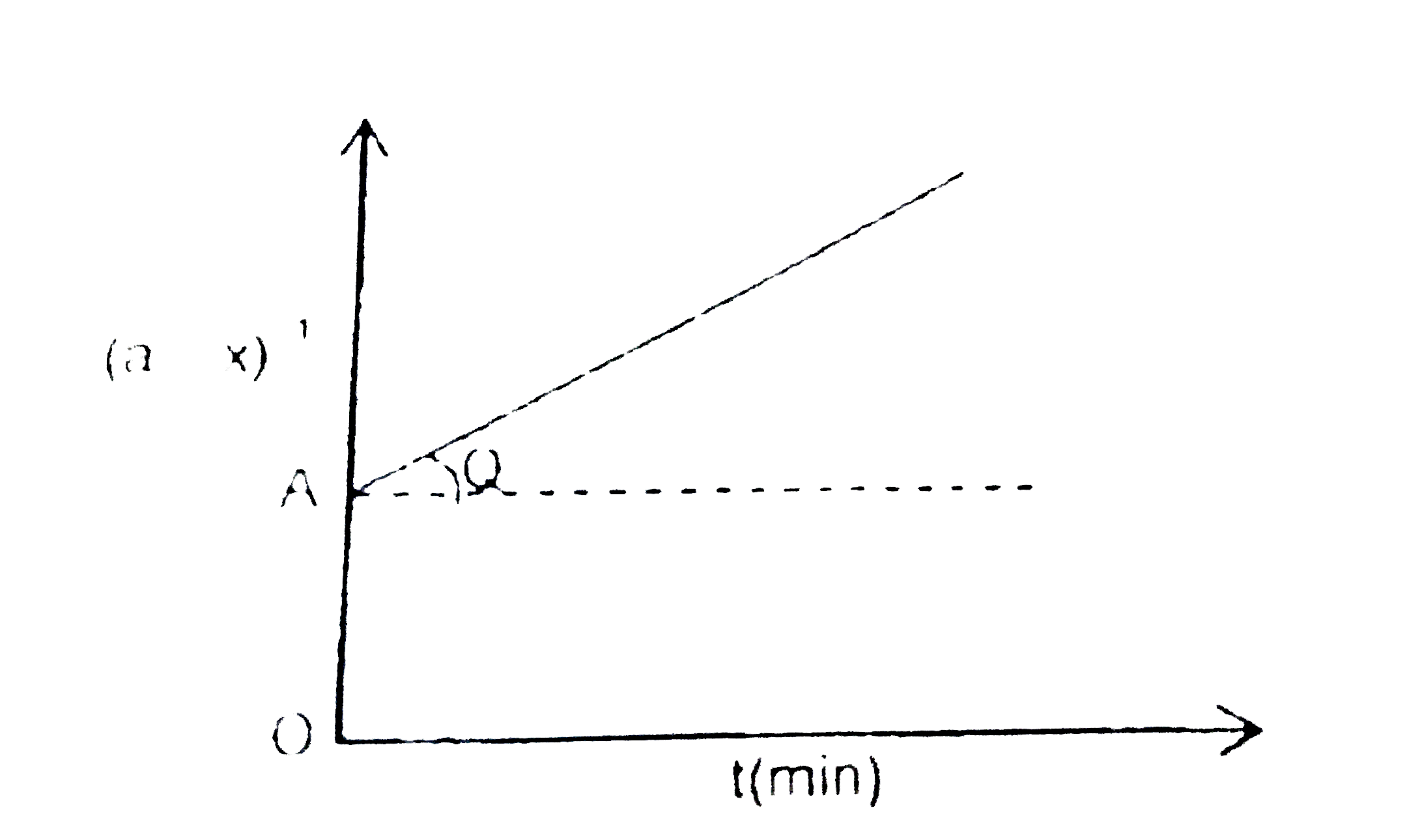
- Following is the graph between (a-x)^(-1) and time for second order re...
Text Solution
|
- Following is the graph between (a-x) and time t for second order react...
Text Solution
|
- Following is the graph between (a-x)^(-1) and time for second order re...
Text Solution
|
- At T (K) , if the rate constant of a first order reaction is 4.606xx10...
Text Solution
|
- Which among the following plots are linear (a -x) is the concentration...
Text Solution
|
- For a zero order reaction, the initial concentration of the reactant =...
Text Solution
|
- Plot a graph of concentration of a reactant at time t, [ (a-x)] agains...
Text Solution
|
- For the second order reaction, concentration (x) of the product at tim...
Text Solution
|
- Which of the following is/are correct for the first order reaction ? (...
Text Solution
|