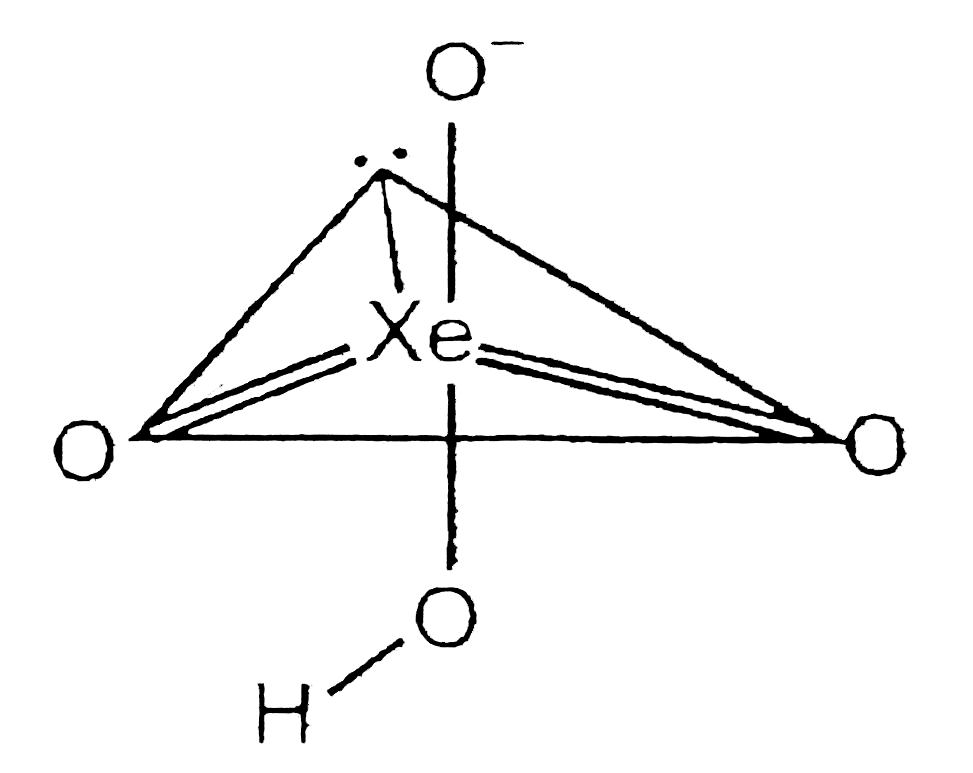
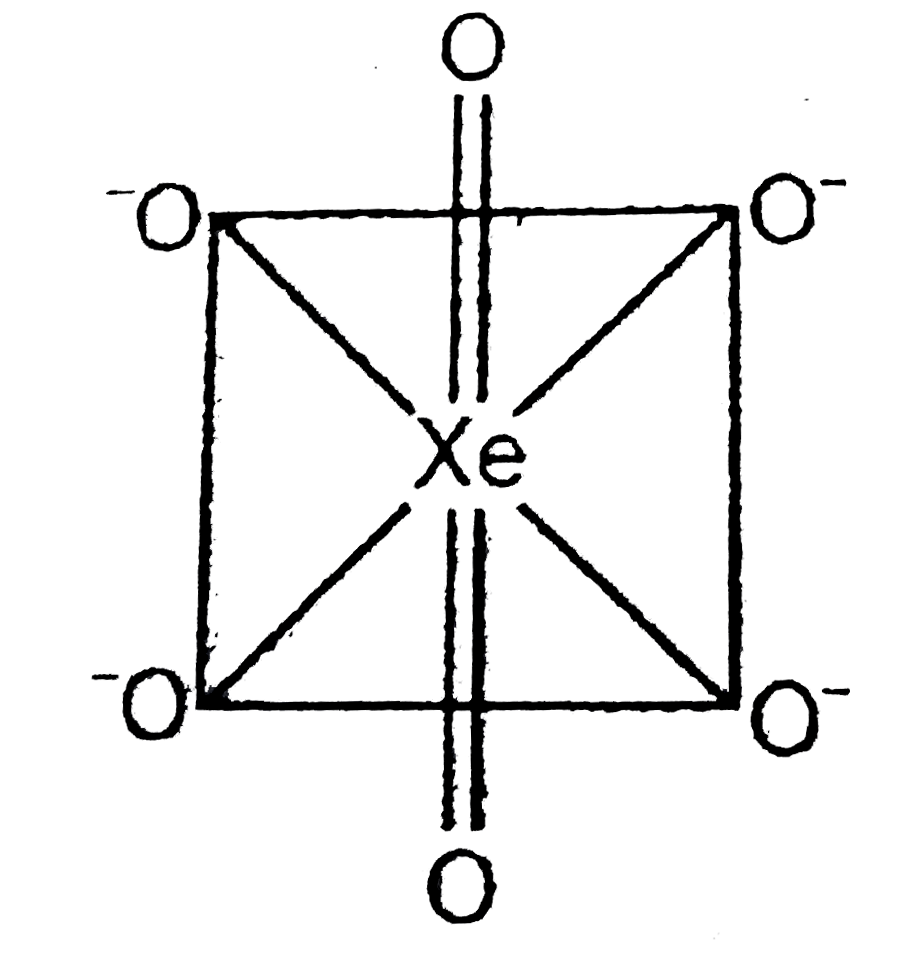
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The xenate ion (HXeO(4)^(-)) undergoes disproportionation reaction to ...
Text Solution
|
- The number of peroxide bonds in perxenate ion [XeO(6)]^(4-) is
Text Solution
|
- Asseration: On dissolution of xenates, [HXeO(4)]^(-) in alkaline solut...
Text Solution
|
- The xenate ion (HXeO(4)^(-)) undergoes disproportionation reaction to ...
Text Solution
|
- The number of pi bonds in perxenate ion is :
Text Solution
|
- XeO(3) forms xenate ion in alkaline medium. XeO(3) +NaOH rarr Na[HXeO(...
Text Solution
|
- Does CIO(4) (perchlorate ion ) undergo disproportionation in slolution...
Text Solution
|
- Perxenate ion is
Text Solution
|
- The correct statement regarding perxenate ion (XeO(6)^(4-)) is:
Text Solution
|