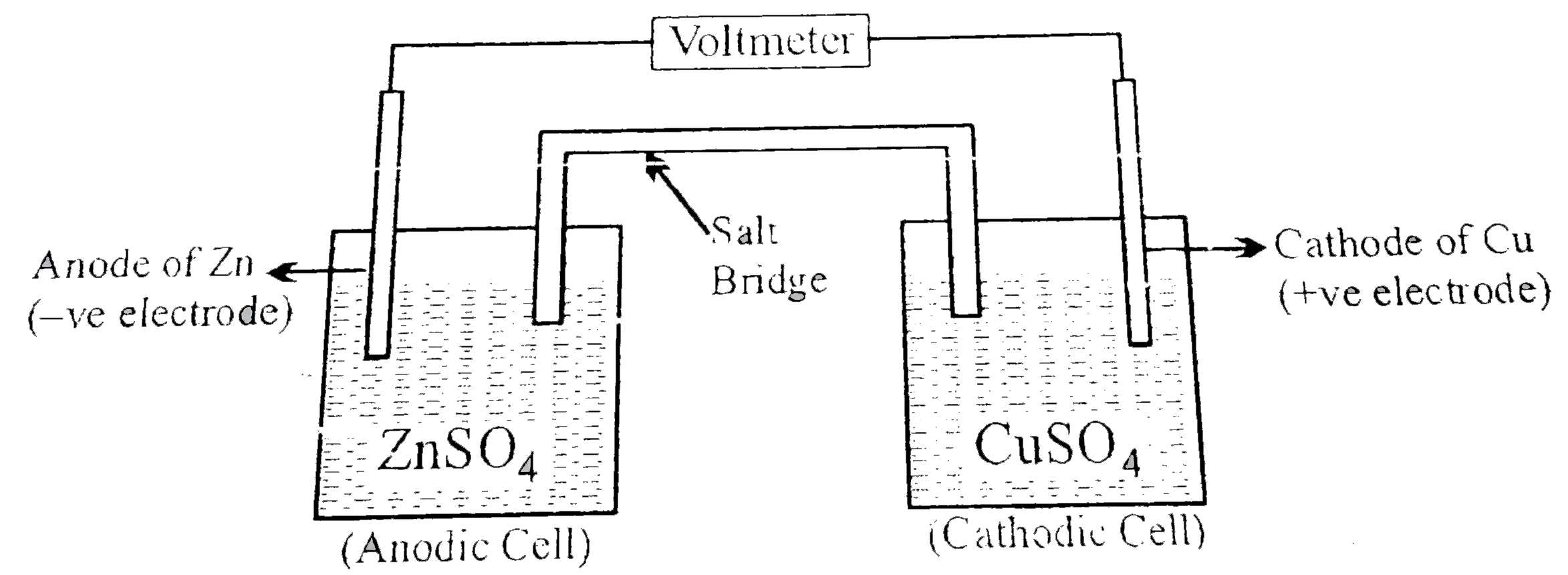A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The most pupular electrochemical cell, daniel cell was originally deve...
Text Solution
|
- The most pupular electrochemical cell, daniel cell was originally deve...
Text Solution
|
- The most pupular electrochemical cell, daniel cell was originally deve...
Text Solution
|
- E^(@)Cu=0.34V,E(Zn)^(@)=-0.76V. A Daniell cell contains 0.1M ZnSO(4) s...
Text Solution
|
- E(Cu^(+2)//Cu)^(@)=0.34V, E(Zn//Zn^(+))^(@)=0.76V A cell formed by t...
Text Solution
|
- For the cell given, below Zn|Zn^(2)||Cu^(2+)|Cu,(E(cell)-E(cell)^(@)...
Text Solution
|
- The emf (in V) of a Daniell cell containing 0.1 M ZnSO(4) and 0.01 M C...
Text Solution
|
- Determine the EMF of a decimolar Daniell cell at 25^(@)C . Given : E(Z...
Text Solution
|
- See whether the cell representation given below is in accordance with ...
Text Solution
|