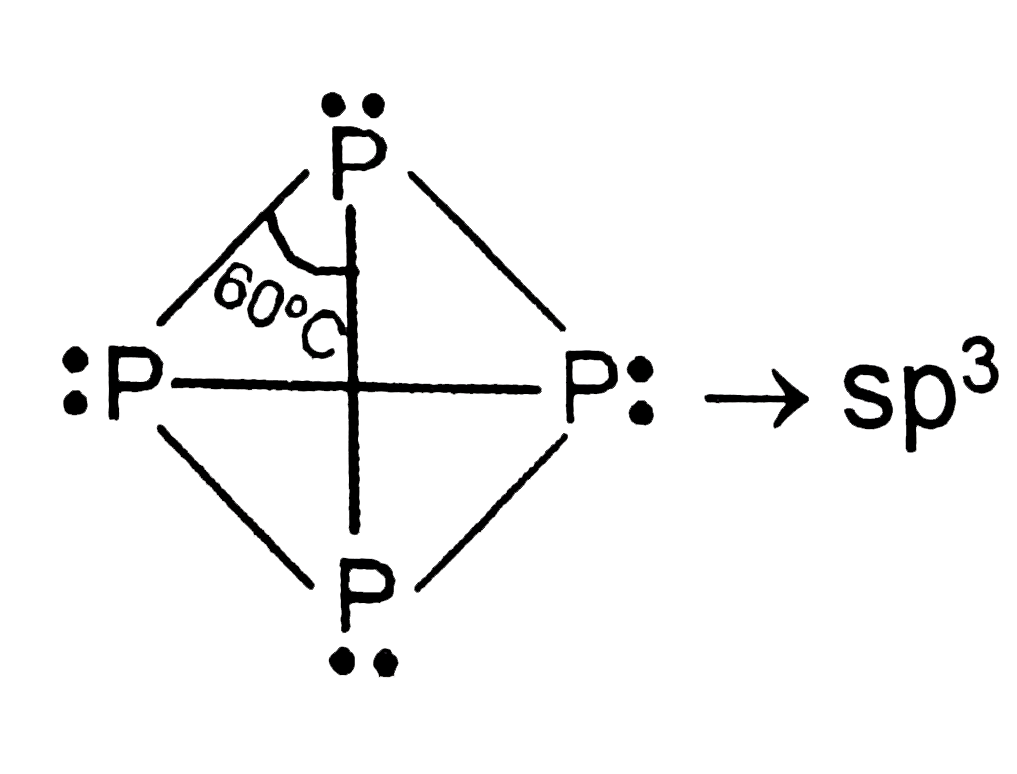
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise Exercise 1 part 3 ASSERTION/REASONING|19 VideosP BLOCK ELEMENTS
RESONANCE|Exercise Exercise 2 part 1 SUBJECTIVE|17 VideosP BLOCK ELEMENTS
RESONANCE|Exercise Exercise 1 part 1 subjective ques|30 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-Exercise 1 part 2 objective que
- Red and white phosphorus will differ but not in:
Text Solution
|
- Compound used in safety matches is :
Text Solution
|
- The high reactivity and low volatility of white phosphorous is due to:
Text Solution
|
- Which of the following oxides is amphoteric in nature?
Text Solution
|
- Which of the following is least reactive?
Text Solution
|
- ….is obtained when ammonium dichromate is heated.
Text Solution
|
- In the ostwald's process, nitric acid is prepared by the catalytic oxi...
Text Solution
|
- NH(4)Cl(s) is heated in test tube.Vapours are brought in contract with...
Text Solution
|
- Which of the following combines with Fe^(2+) ions to form brown comple...
Text Solution
|
- NO(2) can be prepared by heating :
Text Solution
|
- The wrong statement about N(2)O is :
Text Solution
|
- Following are neutral oxides except :
Text Solution
|
- HNO(3)+P(4)O(10) to HPO(3) + X in the above reaction the product X i...
Text Solution
|
- Cold solution of barium nitrite on mixing with sulphuric acid produces...
Text Solution
|
- Nitric acid usually turns yellow on standing.This is due to
Text Solution
|
- Concentrated nitric acid oxidises phosphours (P) into:
Text Solution
|
- Which of the following metals does not dissolve in concentrated HNO(3)...
Text Solution
|
- The compound which has molecular nature is gas phase but ionic in soli...
Text Solution
|
- When P(4)O(10) is dissolved in water, the acid formed finally is :
Text Solution
|
- The substance used as a fast drying agent in the laboratory is:
Text Solution
|