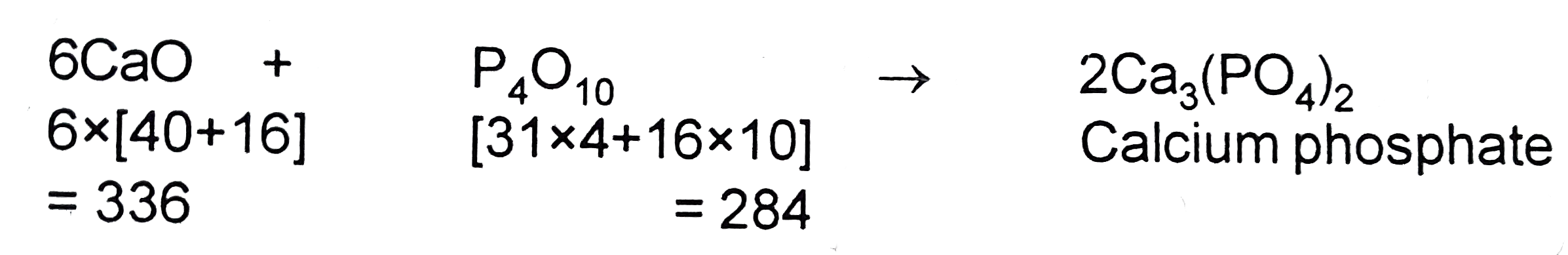Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise ALP PART II AIEEE OBJECTIVE|1 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 2 OBJECTIVE|5 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP EX #3 OBJECTIVE|1 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems