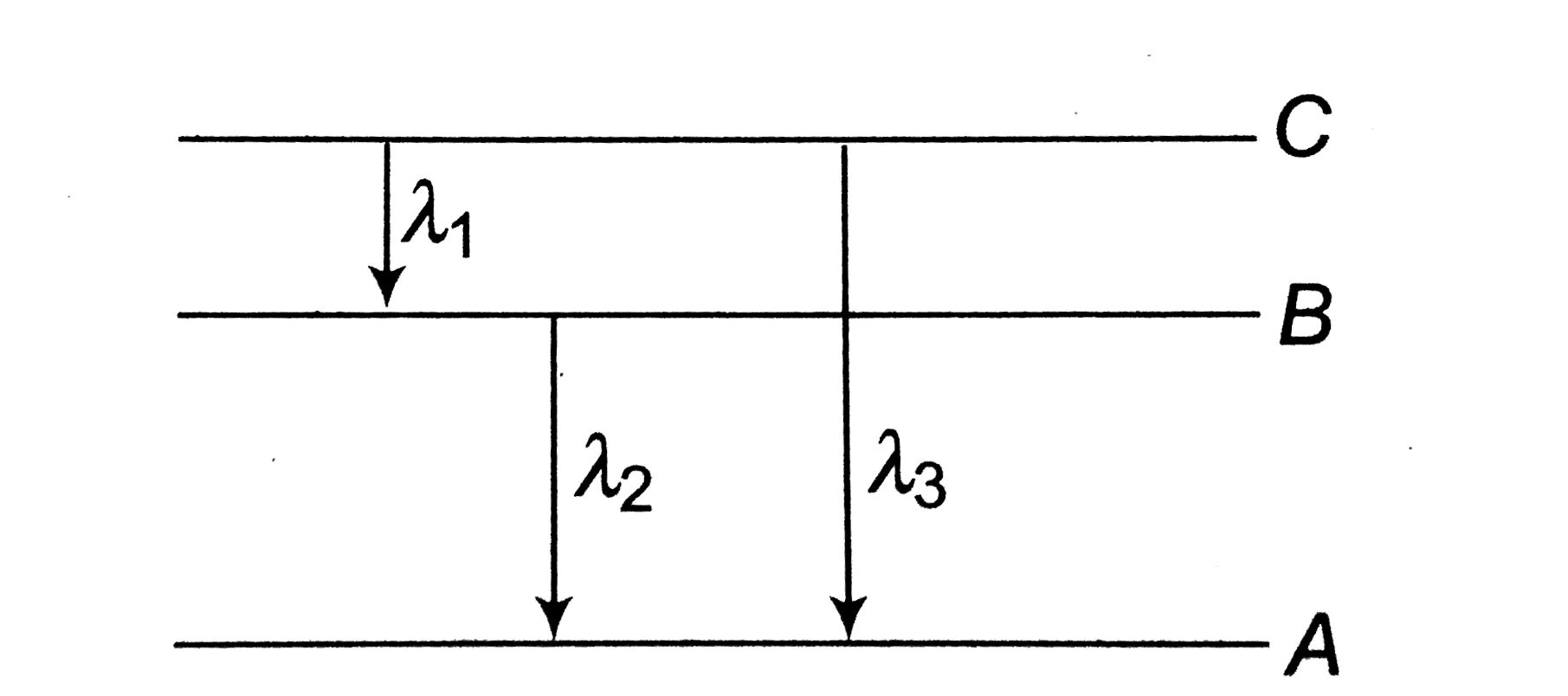
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-ATOMS-PHYSICS
- The Lyman series of hydrogen spectrum lies in the region :
Text Solution
|
- The energy levels of the hydrogen spectrum is shown in figure. There a...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- If m is mass of electron, v its velocity, r the radius of stationary c...
Text Solution
|
- when a hydrogen atom is raised from the ground state to an excited sta...
Text Solution
|
- The ratio of the kinetic energy to the total energy of an eelctron in ...
Text Solution
|
- The ratio of the frequencies of the long wavelenght limits of Lyman an...
Text Solution
|
- Ratio of the wavelength of first line of Lyaman series and first line ...
Text Solution
|
- According to Bohr's theory the moment of momentum of an electron revol...
Text Solution
|
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- Which of the following transition in hydrogen atom emit photons of big...
Text Solution
|
- As par Bohr model, the minimum energy (in eV) required to remove an el...
Text Solution
|
- The third line of Balmer series of an ion equivalent to hydrogen atom...
Text Solution
|
- The wavelength of radiation emitted is lambda(0) when an electron jump...
Text Solution
|
- The energy of electron in the nth orbit of hydrogen atom is expressed ...
Text Solution
|
- Consider a hydrogen-like atom whose energy in nth excited state is giv...
Text Solution
|
- In the Bohr model of the hydrogen atom, let R, v and E represent the r...
Text Solution
|
- An alpha-particle of 5MeV energy strikes with a nucleus of uranium at ...
Text Solution
|
- In a hypotherical Bohr hydrogen, the mass of the electron is doubled. ...
Text Solution
|
- The electron in a hydrogen atom makes a transition n(1) rarr n(2), whe...
Text Solution
|