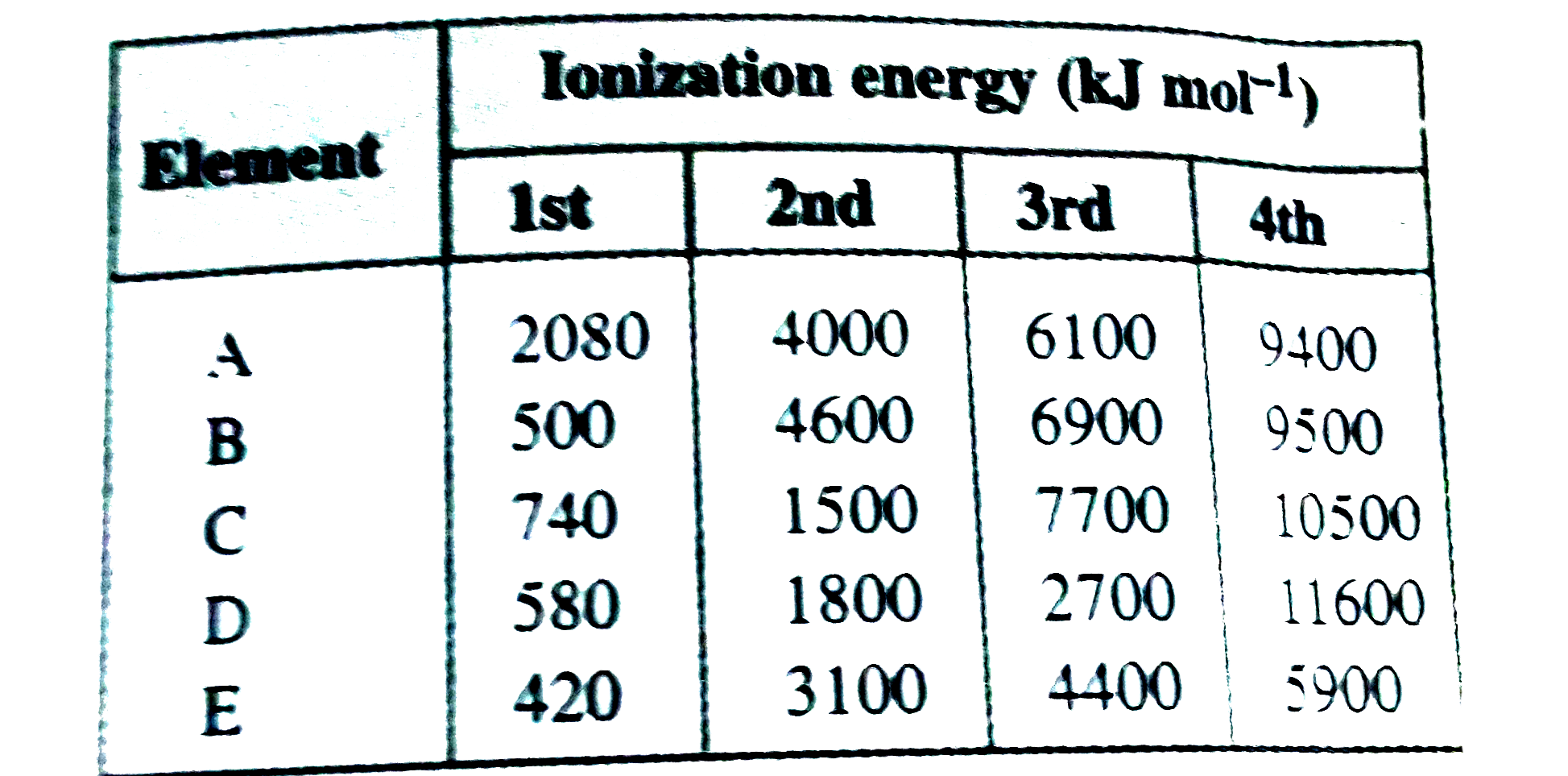
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Multiple Choice Question II )(With one or More than One Correct Answer)|6 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Multiple Choice Question III ) (Based on the given passage / Comprehension ) (Comprehension 1 )|5 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Analytical Question and Problems ( PROBLEMS )|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise Curiosity Questions|2 VideosENVIRONMENTAL CHEMISTRY
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II|6 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Multiple Choice Question I )
- The five successive energies of an element are 800, 2427, 3658, 25024 ...
Text Solution
|
- For one of the element various successive ionization enthalpies ...
Text Solution
|
- The following table shows the successive molar ionization energ...
Text Solution
|
- One mole of magnesium in the vapor state absored 1200 kJ mol^(-1) of e...
Text Solution
|
- Amongest Be, B, Mg and Al, the second ionization potential is maximum ...
Text Solution
|
- The second ionization energies of Li, Be, B and C are in the order
Text Solution
|
- Which of the following processes involves absorption of energy?
Text Solution
|
- Electron affinity is positive when
Text Solution
|
- How does the electron gain enthalpies vary across a period and ...
Text Solution
|
- The first ionisation potential of Na is 5.1eV. The value of electrons ...
Text Solution
|
- Which of the following represents the correct order of increasing elec...
Text Solution
|
- The order of decreasing negative electron gain enthalpy of O. S...
Text Solution
|
- the correct order of electron gain enthalpy with negative sign of F,Cl...
Text Solution
|
- Which of the following atom should have the highest negative first ele...
Text Solution
|
- The element with positive electron gain enthalpy is
Text Solution
|
- Which of the following species has the highest electron affinit...
Text Solution
|
- The highest electron affinity is shown by
Text Solution
|
- The electronegativity of the following elements increases in the orde...
Text Solution
|
- The correct order of electronegativities of N,O, F and P is
Text Solution
|
- Which of the configuration of most electronegative elements is
Text Solution
|