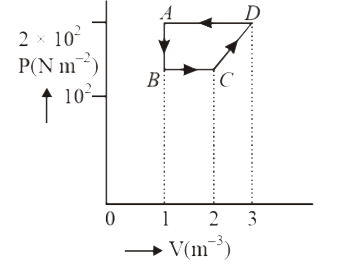
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-THERMODYNAMICS-PHYSICS
- A mass of diatomic gas(gamma=1.4) at a pressure of 2 atomphere is comp...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- The P-V diagram of a gas system undergoing cyclic process is shown her...
Text Solution
|
- During an adiabatic process of an ideal gas, if P is proportional to (...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- Consider a spherical shell of radius R at temperature T. The black bod...
Text Solution
|
- The specific heat capacity of a metal at low temperature (T) is given ...
Text Solution
|
- 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 lite...
Text Solution
|
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- In a adiabatic process pressure is increased by 2//3% if C(P)//C(V) = ...
Text Solution
|
- A reversible engine converts one-sixth of the heat input into work. Wh...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|
- When the state of a gas adiabatically changed from an equilibrium stat...
Text Solution
|
- Calculate the work done when one mole of a perfect gas is compressed a...
Text Solution
|
- An ideal Carnot's engine whose efficiency 40% receives heat of 500K. I...
Text Solution
|
- One kg of water at 373K is converted into steam at the same temperatur...
Text Solution
|
- One mole of an ideal gas at temperature T was cooled isochorically til...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- One litre of ideal gas is conpressed isothermally at 0.72m of Hg-colum...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|