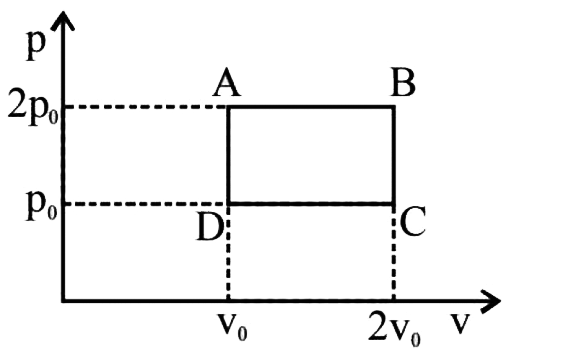
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-THERMODYNAMICS-PHYSICS
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- An ideal gas undergoing adiabatic change has the following pressure-te...
Text Solution
|
- In a thermodynamic process, pressure of a fixed mass of a gas is chang...
Text Solution
|
- The cofficient of performance of a refrigerator is 5. If the temperatu...
Text Solution
|
- Two gases have the same initial pressure, volume and temperature. They...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- For an ideal gas graph is shown for three processes. Process 1, 2 and ...
Text Solution
|
- During an adiabatic process an object does 100J of work and its temper...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- A perfect gas goes from a state A to another state B by absorbing 8 × ...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- Two Carnot engines A and B are operated in series. The engine A receiv...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands to P2,V2 and then compre...
Text Solution
|
- Which of the following statements is correct for any thermodynamic sys...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- A sample of an ideal gas in a cyclinder is compressed adiabatically to...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- In the P-V diagram shown in figure ABC is a semicircle. The work done ...
Text Solution
|
- For an isothermal expansion of a perfect gas, the value of (DeltaP)/(P...
Text Solution
|