A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-KINETIC THEORY OF GASES-PHYSICS
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
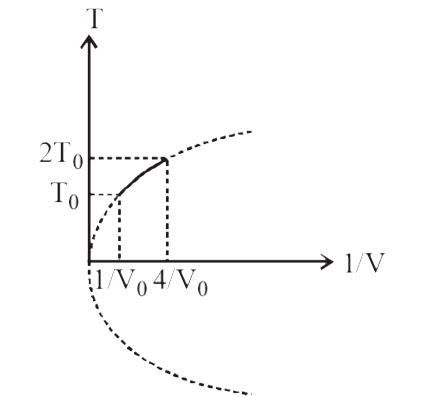
- Figure shows a parabolic graph between T and 1/V for a mixture of a ga...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- At what temperature is the root mean square velocity of gaseous hydrog...
Text Solution
|
- The kinetic theory of gases states that the average squared velocity o...
Text Solution
|
- If 2 mol of an ideal monatomic gas at temperature T(0) are mixed with ...
Text Solution
|
- From the following statements concerning ideal gas at any given temper...
Text Solution
|
- Figure shows the pressure P versus volume V graphs for a certains mass...
Text Solution
|
- The molecules of a given mass of a gas have rms velocity of 200 m//s a...
Text Solution
|
- A graph is plotted with PV/T on y-axis and mass of the gas along x-axi...
Text Solution
|
- At identical temperatures, the rms speed of hydrogen molecules is 4 ti...
Text Solution
|
- Find the expression for the work done by a system undergoing isotherma...
Text Solution
|
- Two vessel separately contains two ideal gases A and B at the same tem...
Text Solution
|
- The temperature of the mixture of one mole of helium and one mole of h...
Text Solution
|
- If the intermolecules forces vanish away, the volume occupied by the m...
Text Solution
|
- If the intermolecules forces vanish away, the volume occupied by the m...
Text Solution
|
- A vessel has 6g of hydrogen at pressure P and temperature 500K. A smal...
Text Solution
|
- For a gas if ratio of specific heats at constant pressure and volume i...
Text Solution
|
- The given P-V curve is predicted by
Text Solution
|