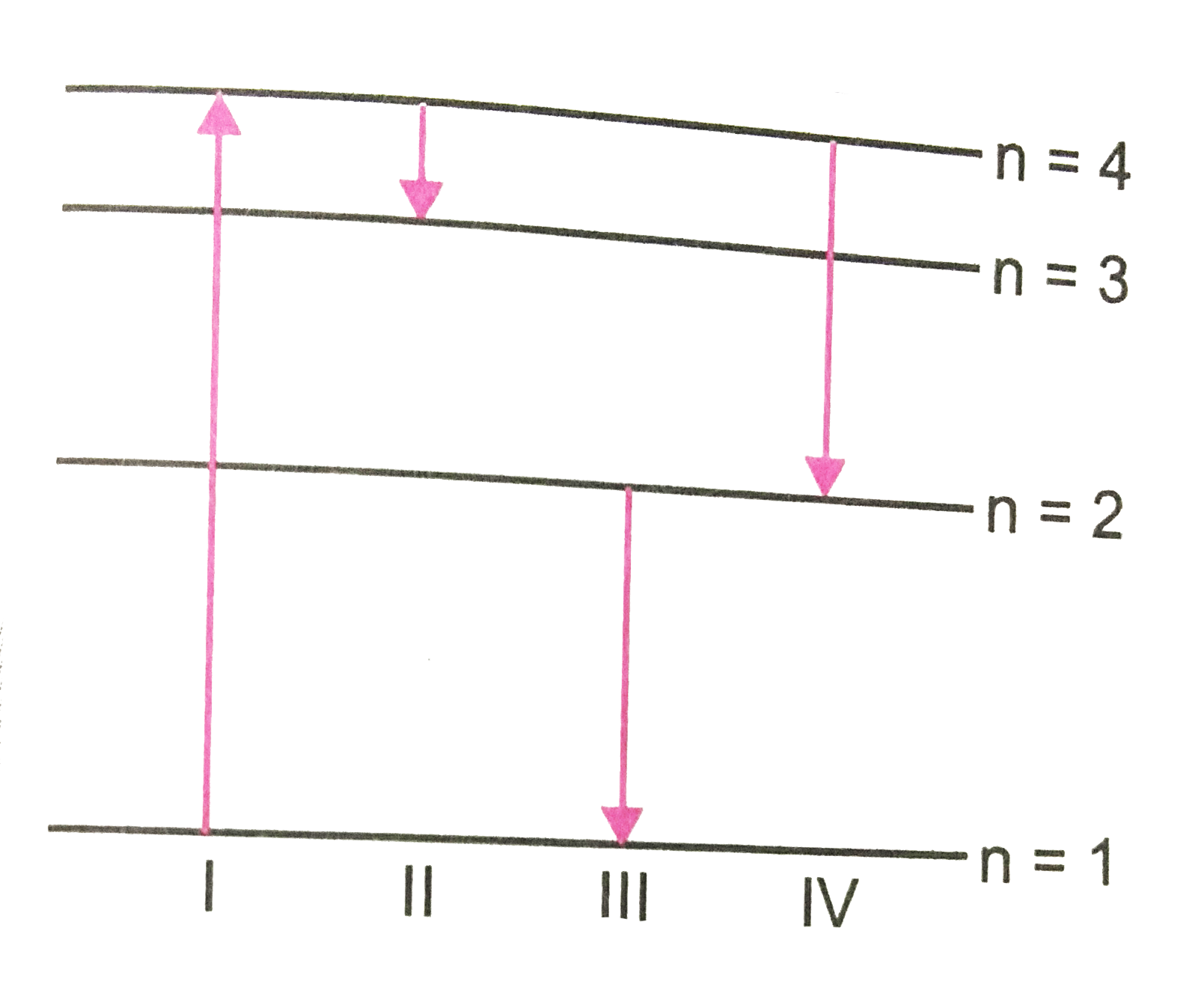
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-ATOMS-PHYSICS
- Assertion: Hydrogen atom consists of anly one electron but its emissio...
Text Solution
|
- The potential energy associated with an electron in the orbit
Text Solution
|
- Shows the energy levels for an electron in a certain atom. Which trans...
Text Solution
|
- Electrons in a certain energy level n=n(1) can emit 3 spectral lines. ...
Text Solution
|
- In Rutherford scattering experiment, the number of alpha-particles sca...
Text Solution
|
- In the Bohr model an electron moves in a circular orbit around the pro...
Text Solution
|
- A 12.5 eV electron beam is used to excite a gaseous hydrogen atom at r...
Text Solution
|
- A hydrogen atom and a Li^(2+) ion are both in the second excited stat...
Text Solution
|
- The radius of hydrogen atom in its ground state is 5.3 xx 10^(-11)m. A...
Text Solution
|
- When hydrogen atom is in first excited level, its radius is….its groun...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- An electron in the hydrogen atom jumps from excited state n to the gro...
Text Solution
|
- The electron in a hydrogen atom makes a transition from an excited sta...
Text Solution
|
- As energy of 24.6 eV is required to remove one of the required to remo...
Text Solution
|
- One of the lines in the emission spectrum of Li^(2+) has the same wave...
Text Solution
|
- If the atom(100)Fm^(257) follows the Bohr model the radius of (100)Fm^...
Text Solution
|
- The energy of He+ in the ground state is -54.4 eV, then the energy of ...
Text Solution
|
- If the angular momentum of an electron in an orbit is J then the K.E. ...
Text Solution
|
- Suppose an electron is attracted toward the origin by a force(k)/(r ) ...
Text Solution
|
- In hydrogen spectrum the wavelength of H(a) line is 656nm, where in t...
Text Solution
|