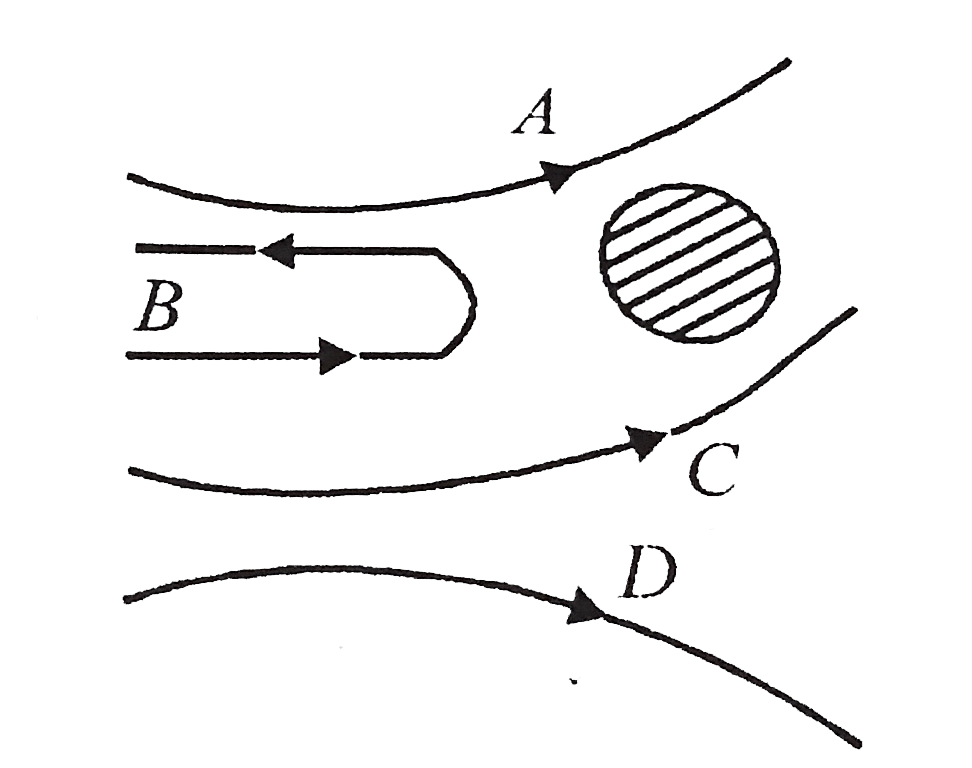
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA-ATOMS-PHYSICS
- In a hydrogen like atom electron make transition from an energy level ...
Text Solution
|
- The spectrum obtained from a sodium vapour lamp is an example of
Text Solution
|
- Ionization potential of hydrogen atom is 13.6 V. Hydrogen atoms in the...
Text Solution
|
- The Bohr model of atoms
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- A doubly ionised Li atom is excited from its ground state(n = 1) to n ...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- The ionisation energy of hydrogen atom is 13.6 eV. Following Bohr's th...
Text Solution
|
- The transition form the state n = 3 to n = 1 in a hydrogen-like atom r...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- Which of the plots shown in the figure represents speed (vn) of the el...
Text Solution
|
- The ionisation potential of H-atom is 13.6 eV. When it is excited from...
Text Solution
|
- If the energy of a hydrogen atom in nth orbit is E(n), then energy in ...
Text Solution
|
- In the Rutherford experiment, alpha-particles are scattered from a nuc...
Text Solution
|
- An electron changes its position from orbit n = 4 to the orbit n = 2 o...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- The wavelength of the first spectral line in the Balmer series of hydr...
Text Solution
|
- Let v(1) be the frequency of series limit of Lyman series, v(2) the fr...
Text Solution
|
- In a hypotherical Bohr hydrogen, the mass of the electron is doubled. ...
Text Solution
|