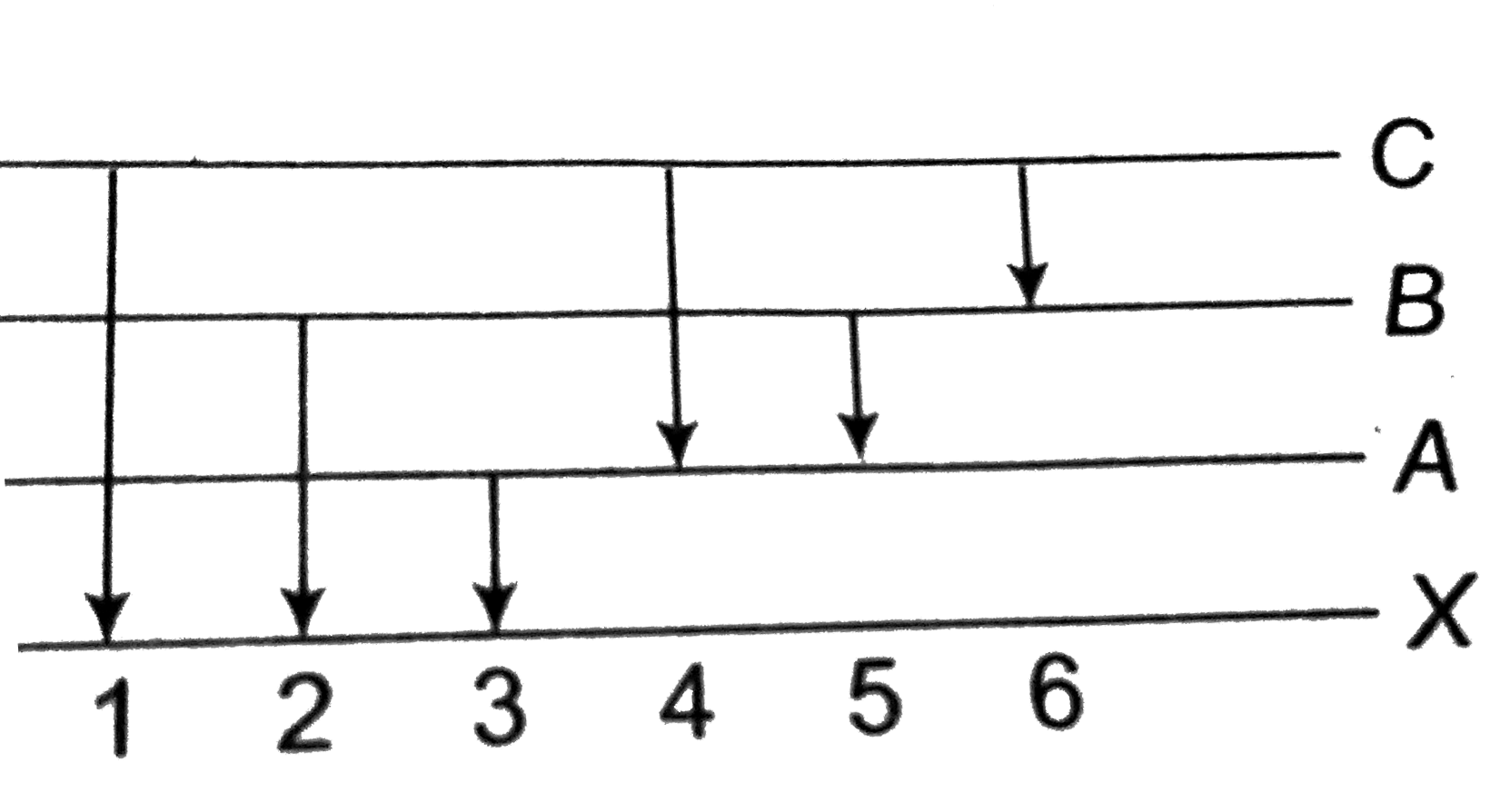
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-MODERN PHYSICS-PHYSICS
- Electrons accelerated from rest by a potential difference of 12.75V, a...
Text Solution
|
- Three photons coming from excited atoms hydrogen sample are picked up ...
Text Solution
|
- The figure indicates the enegry level diagram of an atom and the origi...
Text Solution
|
- When a potential drop of V volt is applied on a stationary neutron. th...
Text Solution
|
- When an electron moving with constant speed enters in a magnetic field...
Text Solution
|
- The de Broglie wave present in fifth Bohr orbit is:
Text Solution
|
- A nucleus at rest undergoes a decay emitting an alpha -particle of de-...
Text Solution
|
- In previous question KE of daughter nucleous is -
Text Solution
|
- An electron is confined to a tube of length L. The electron’s potentia...
Text Solution
|
- An electron moving with a velocity of 10^(6) m//s in the X-direction ...
Text Solution
|
- If the thermal energy of a monoatomic gas molecules of mass m at tempe...
Text Solution
|
- The collector plate in an experiment of photoelectric effect is kept v...
Text Solution
|
- A surface does not eject electrons when illuminated with blue light. T...
Text Solution
|
- If work function of a metal is 2 eV and light of 1 eV is incident on t...
Text Solution
|
- A cesium photocell, with a steady potential difference of 60 V across ...
Text Solution
|
- Light corresponding in the transition n = 4 to n = 2 in hydrogen atoms...
Text Solution
|
- When light of sufficiently high frequency is incident on a metallic su...
Text Solution
|
- A non-monochromatic light is used in an experiment on photoelectric ef...
Text Solution
|
- A freshly cleaned zinc plate is connected to the top of a positively c...
Text Solution
|
- The frequency and intensity of a light source are both doubled. Consid...
Text Solution
|