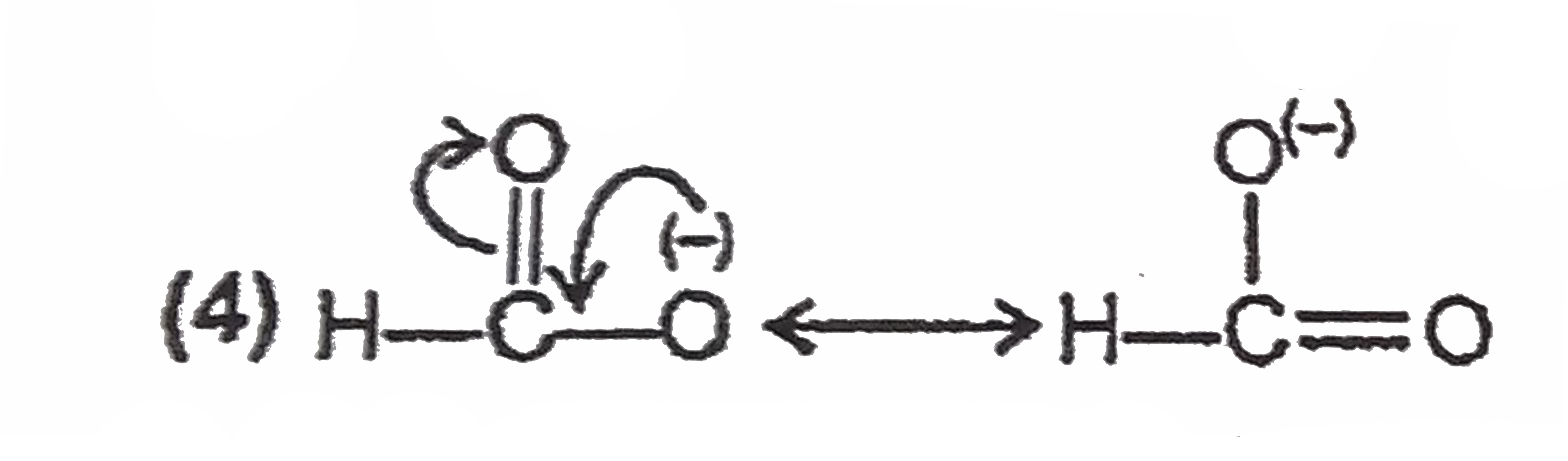
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-FUNDAMENTAL CONCEPT-ORGANIC CHEMISTRY(Fundamental Concept )
- Which compound give CO(2) with NaHCO(3) ?
Text Solution
|
- Correct order of basic nature is
Text Solution
|
- In the anion HCOO^- , the carbon-oxygen bonds are found to be of equal...
Text Solution
|
- Which of the following orders of acid strength is correct?
Text Solution
|
- C(2)H(5)OHoverset(X)rarrC(2)H(5)Cl X can be except :
Text Solution
|
- The conversion of Cl-CH=CH-Cl to CHCl(2)-CHCl(2) can be carried out wi...
Text Solution
|
- In which of the following rate of S(N) 1 is very fast ?
Text Solution
|
- Which is the correct order for basicity ?
Text Solution
|
- Pyridine is less basic than triethylamine because .
Text Solution
|
- The decreasing order of the stability of the ions : underset((I))(CH...
Text Solution
|
- Which order is correct for both nucleophilicity and basicity ?
Text Solution
|
- Arrange pH of the given compounds in decreasing order :
Text Solution
|
- Maximum enol content is in :
Text Solution
|
- Consider the S(N) 1 solvolysis of the following hallides in aqueous fo...
Text Solution
|
- In the following groups: (I) -OAc, (II) -OMe, (III) -OSO(2)Me, (IV) ...
Text Solution
|
- The chemical system that is non- aromatic is
Text Solution
|
- Which of the following is the least stable carbanion?
Text Solution
|
- Arrange stability of the given carbocation in decreasing order :
Text Solution
|
- Which of the following compound give solvolysis reaction with slowest ...
Text Solution
|
- Which of the following alkoxides is the most reactive nucleophile?
Text Solution
|