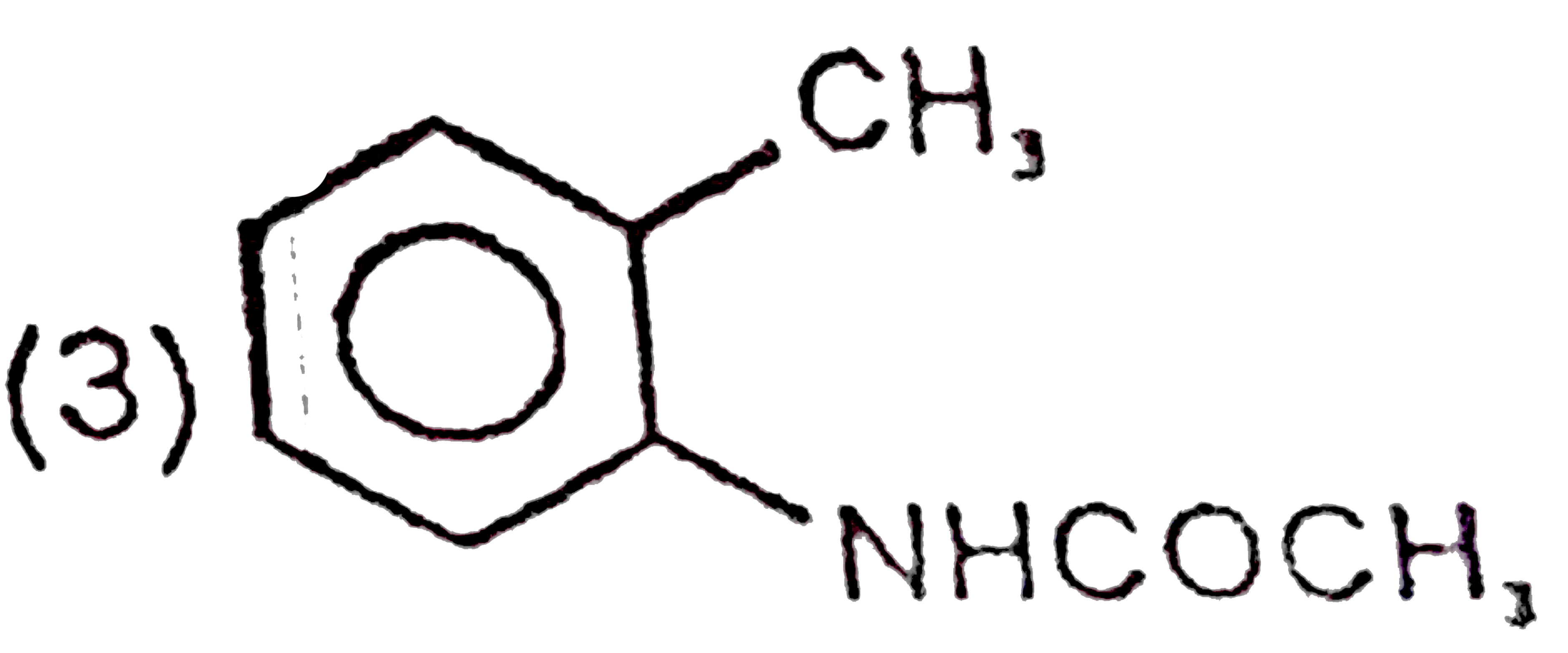
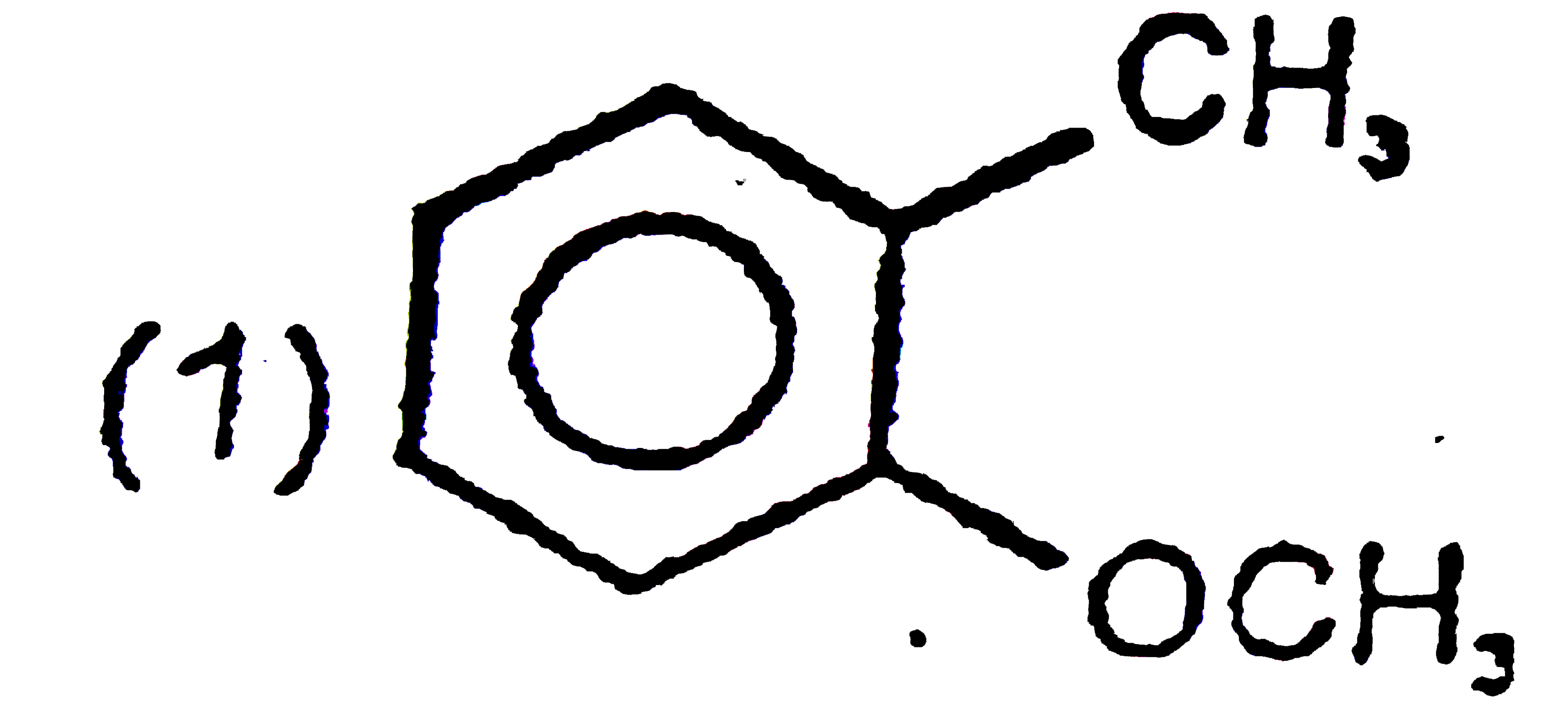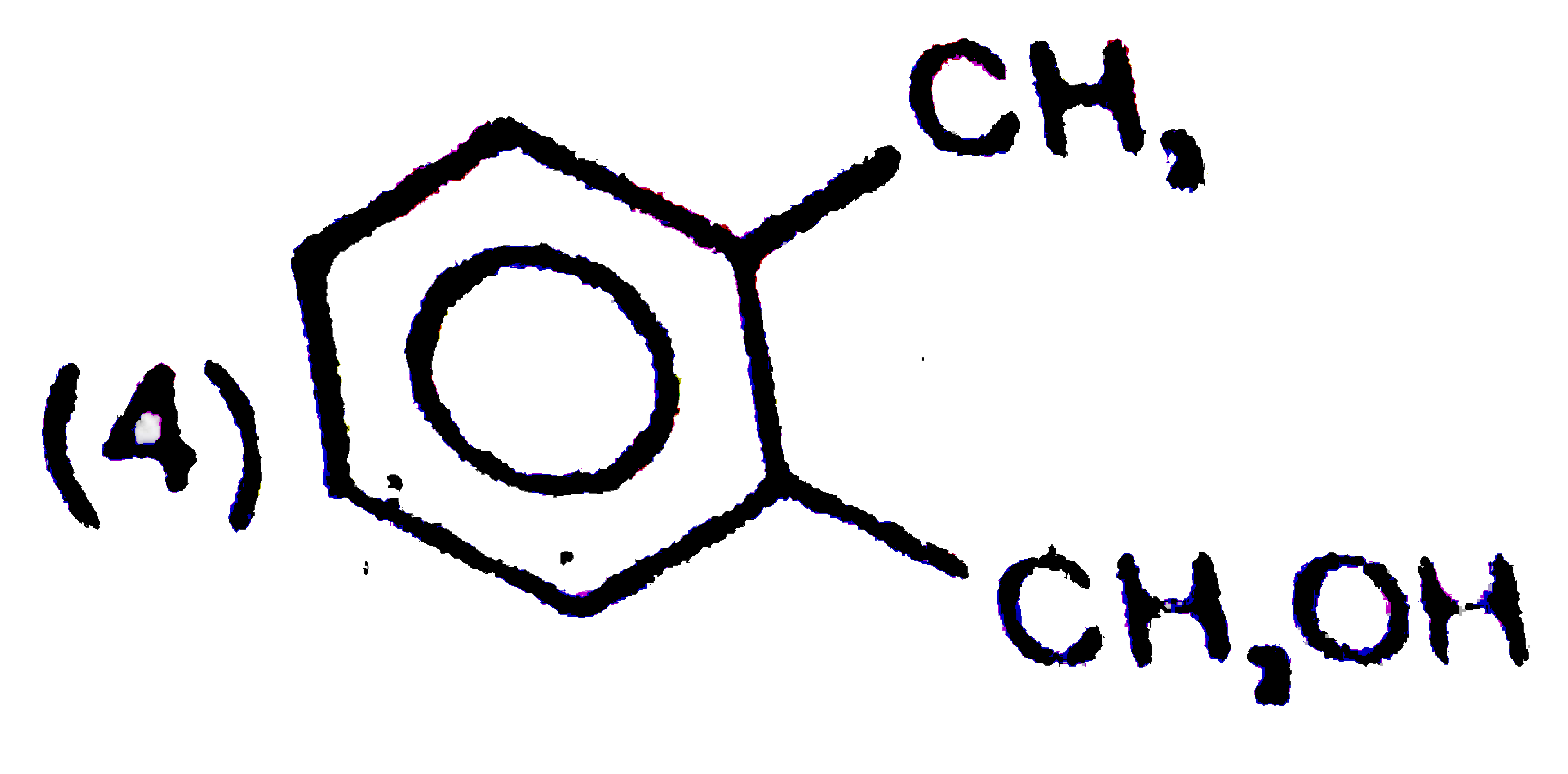A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-HYDROCARBON-ORGANIC CHEMISTRY(Hydrocarbon)
- Benzene reacts with CH(3)Cl in the presence of anyhydrous AlCl(3) to f...
Text Solution
|
- Petroleium mainly contains :
Text Solution
|
- Which one of the following is most reactive towards electrophilic reag...
Text Solution
|
- CH3C-=C CH3underset(CaCO3 and BaSO4)overset((H2)/(Pd))to P Identifty t...
Text Solution
|
- The alkyl halides that can be made by free radical halogenation of alk...
Text Solution
|
- The species which acts as electrophile in the bromination of benzene i...
Text Solution
|
- Reaction by which Banzaldehyde cannot be prepared?
Text Solution
|
- Which of the following carbides on treatment with water give methane ...
Text Solution
|
- Propene, CH(3)-CH = CH(2), can be converted to 1-propanol by oxidation...
Text Solution
|
- What products are formed when the following compounds are treated with...
Text Solution
|
- When HCl gas is passed through propene in the presence of benzoyl pero...
Text Solution
|
- Which of the following is the most reactive towards ring nitration ?
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|
- The product 'Y' in the following reaction sequence is :
Text Solution
|
- Which of the following has the higest knocking property ?
Text Solution
|
- C(6)H(5)Cl+CH(3)Cloverset(Na//"dry ether")rarrC(6)H(5)CH(3)+2NaCl Th...
Text Solution
|
- The reaction CH(3)COCH(3)underset((ii)KOH,"glycol")overset((i)NH(2)-NH...
Text Solution
|
- Type of reactions shown by alkynes are -
Text Solution
|
- Which of the following produces one mononitro and three isomeric dinit...
Text Solution
|
- CH(3)-C equiv C-CH(3)overset(Na//NH(3)(i))rarrPoverset(OH(2)l(2).Zn,De...
Text Solution
|