Similar Questions
Explore conceptually related problems
Recommended Questions
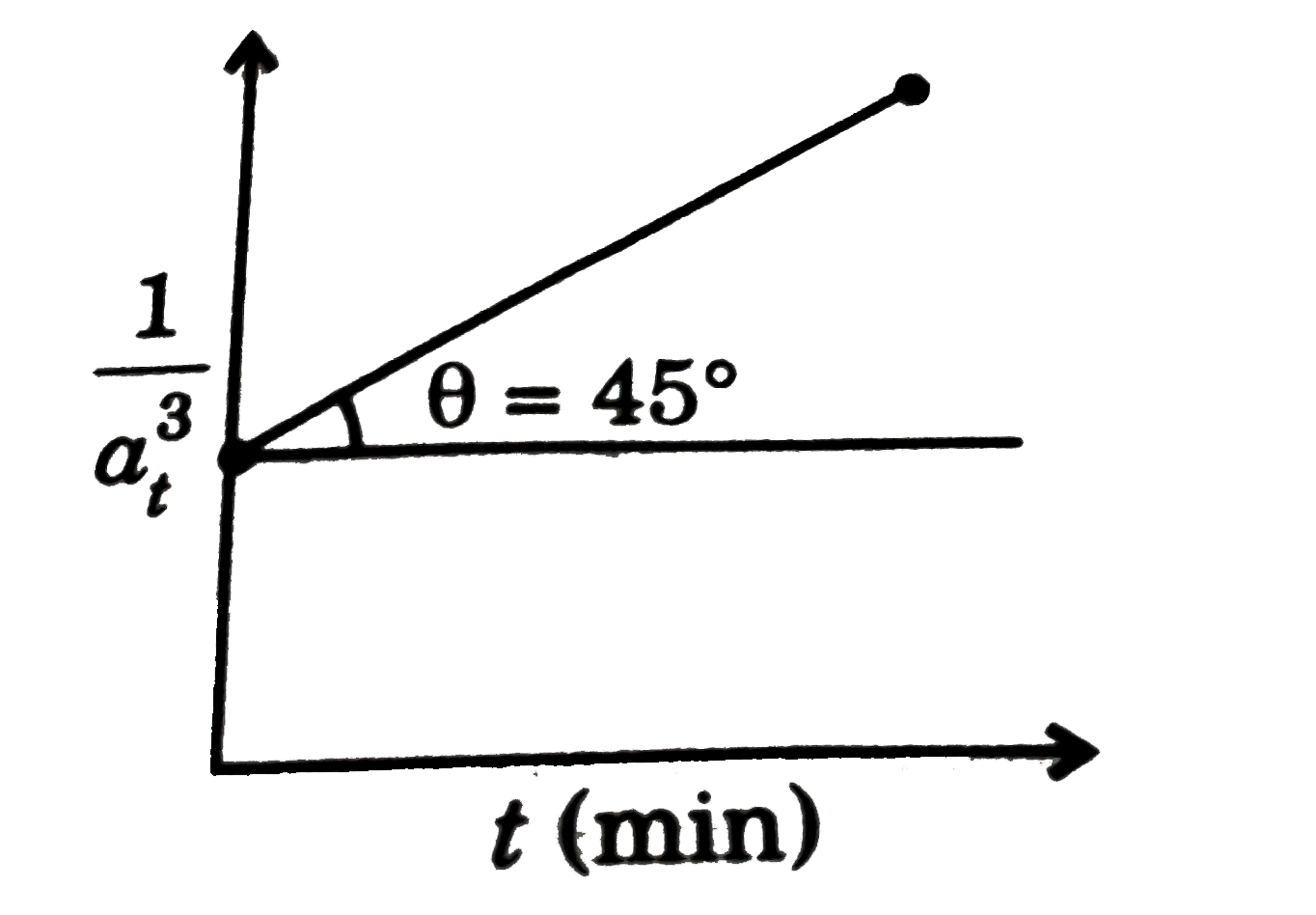
- For a reaction 3A rarr 2B, following graph is obtained, calculate rate...
Text Solution
|
- For a reaction 3A rarr Products, it is found that the rate of reaction...
Text Solution
|
- The rate of the reaction 3A + 2B rarr Products is given by the rate ex...
Text Solution
|
- For a reaction, 3A rarr Products, it is found that the rate of reactio...
Text Solution
|
- If 3A rarr 2B, then the rate of reaction of + (dB)/(d t) is equal to
Text Solution
|
- For a first order reaction: A(3) rarr 3A, Following graph is observed ...
Text Solution
|
- For a reaction 3A rarr 2B, following graph is obtained, calculate rate...
Text Solution
|
- The equilibrium constant for the following reaction will be 3A+2B ra...
Text Solution
|
- The following rate date were obtained at 313 K for the reaction : 2A...
Text Solution
|