Similar Questions
Explore conceptually related problems
Recommended Questions
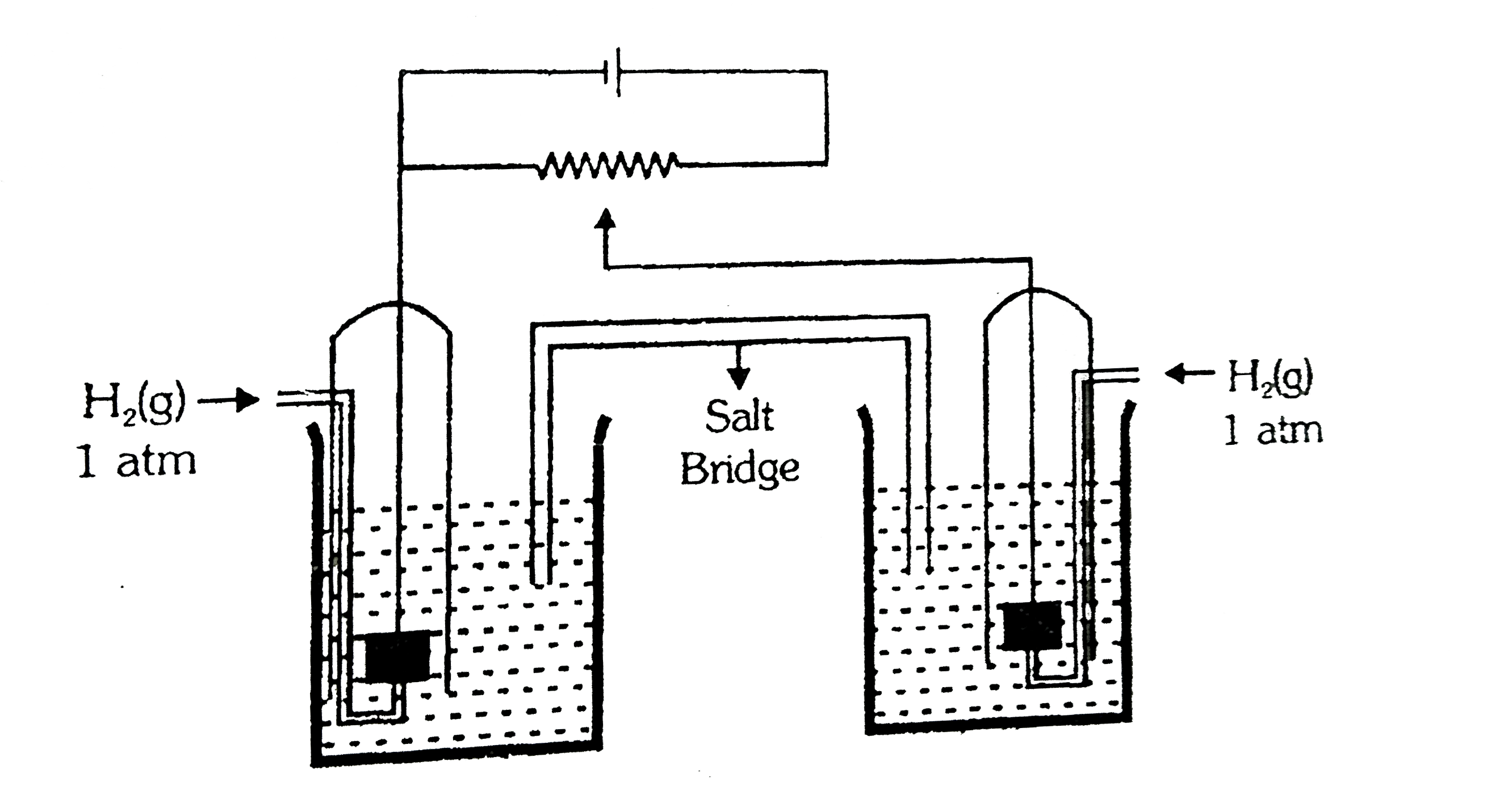
- Carefully observe the given figure and using data provided find the EM...
Text Solution
|
- Calculate the pH of a solution of given mixture. a. (2g CH(3)COOH +3g ...
Text Solution
|
- A solution contains 0.09 HC1, 0.09 M CHC1(2)COOH , and 0.1M CH(3)COOH ...
Text Solution
|
- Calculate the e.m.f. of cell {:(Pt(H(2))),(1atm):}|{:(CH(3)COOH),(0.1M...
Text Solution
|
- Carefully observe the given figure and using data provided find the EM...
Text Solution
|
- % hydrolysis of 0.1M CH(3)COONH(4), when K(a)(CH(3)COOH)=K(b)(NH(4)OH)...
Text Solution
|
- What is the concentration of CH(3)COOH(aq.) in a solution prepared by ...
Text Solution
|
- Dissociation constant of CH(3)COOH and NH(4)OH in squeous solution are...
Text Solution
|
- Consider the following solutions of equal concentrations {:(A= NH(4...
Text Solution
|