Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT-KINETIC THEORY OF GASES-Level 3
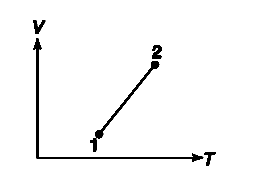
- Two states – 1 and 2 – of an ideal gas has been shown in VT graph. In ...
Text Solution
|
- An ideal gas is inside a cylinder with a piston that can move freely. ...
Text Solution
|
- The speed distribution of molecules in a sample of a gas is shown in t...
Text Solution
|
- A cylindrical container is divided into three parts by two tight fitti...
Text Solution
|
- A helium balloon has its rubber envelope of weight w("Rubber") and its...
Text Solution
|