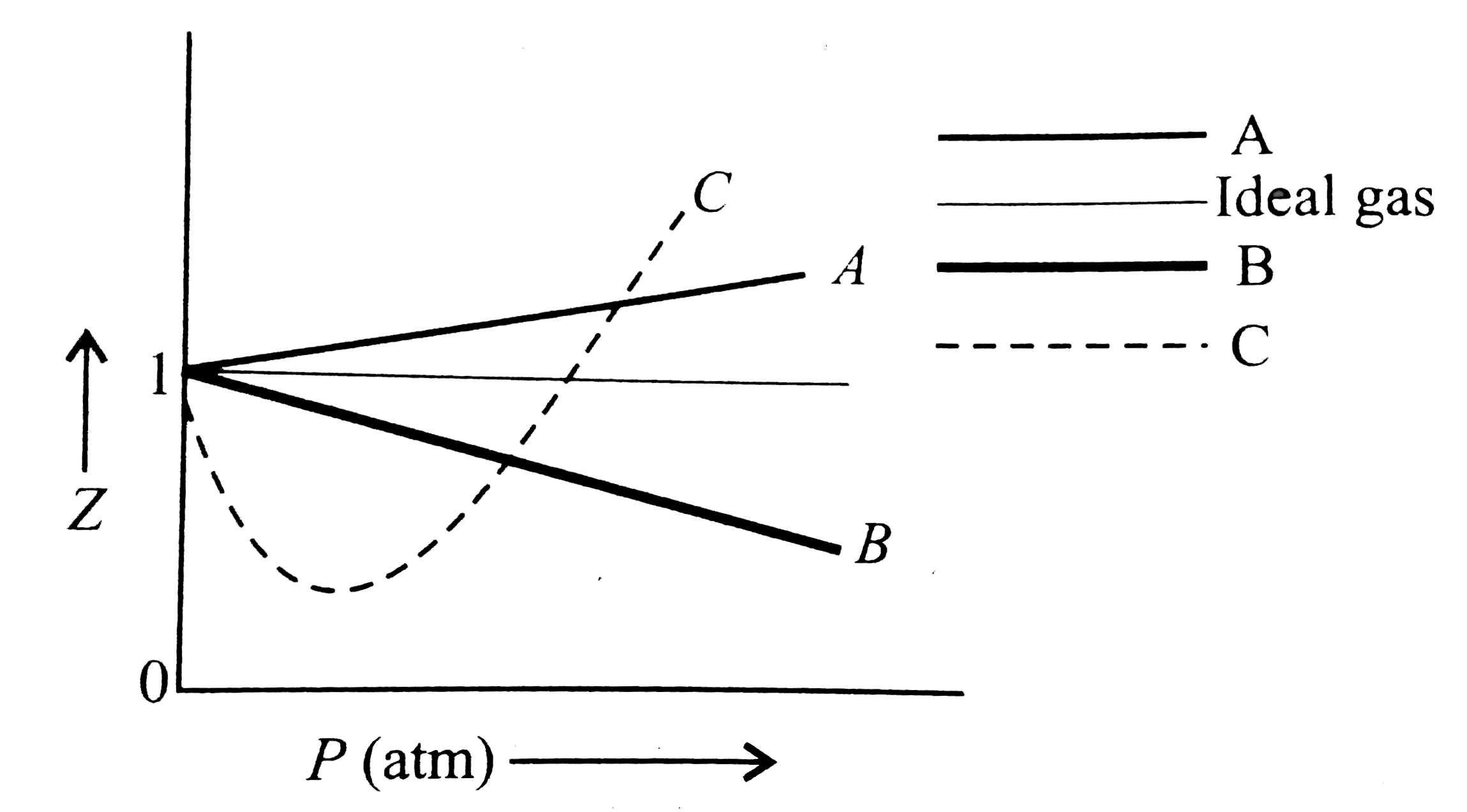
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise OBJECTIVE_TYPE|9 VideosSTATES OF MATTER
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise MATCH THE COLUMN|1 VideosSOME BASIC CONCEPTS OF CHEMISTRY
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Subjective Questions|2 VideosSURFACE CHEMISTRY
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise JEE Main And Advanced|17 Videos
Similar Questions
Explore conceptually related problems