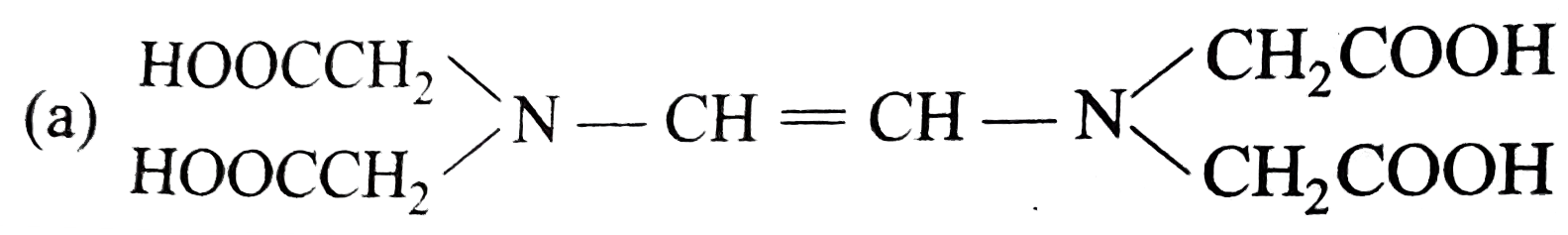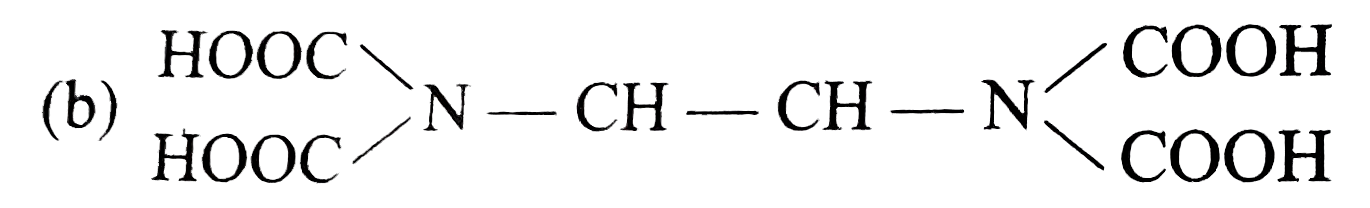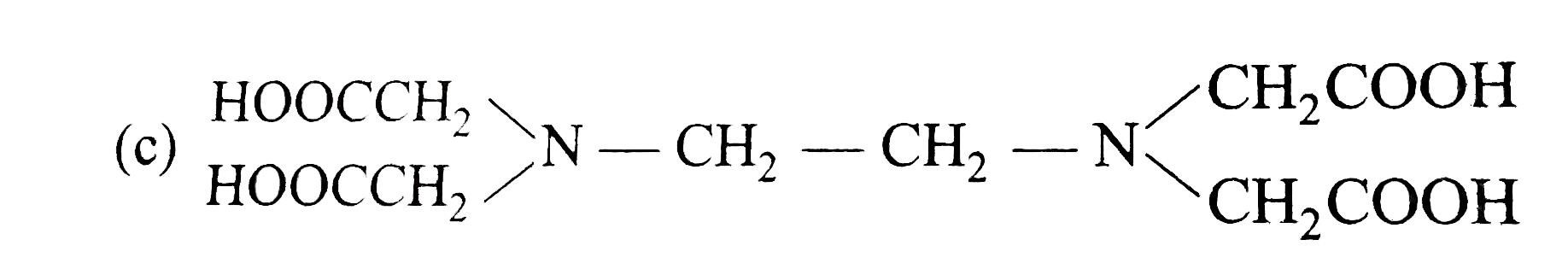A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-COORDINATION COMPOUNDS-PHYSICS
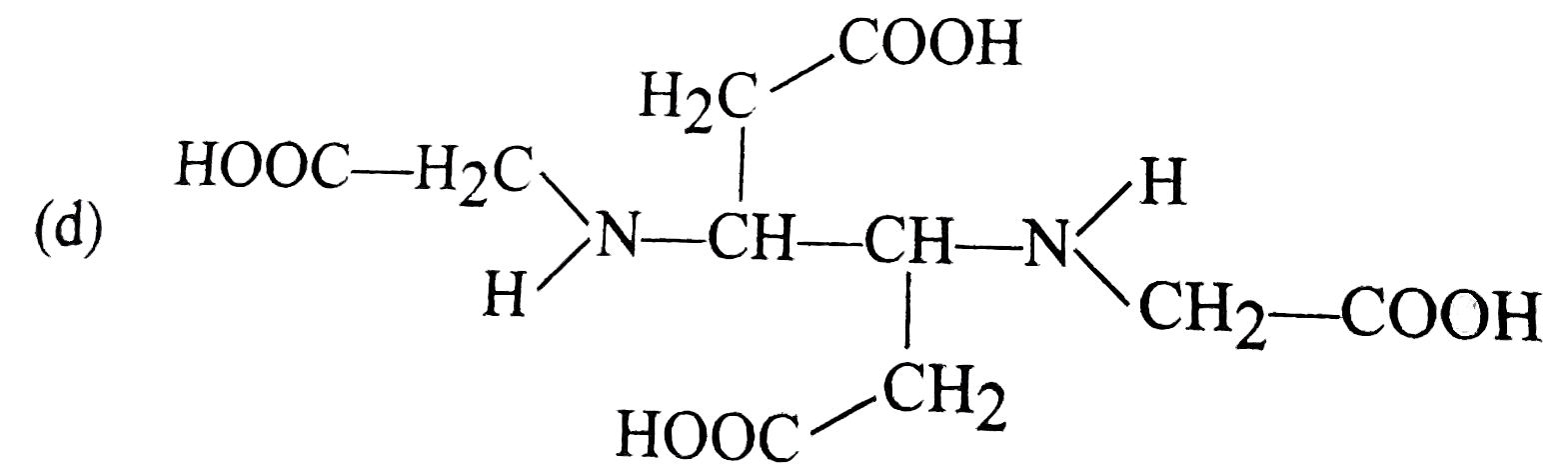
- The correct structure of ethylenediamineteraacetic acid (EDTA) is .
Text Solution
|
- Addition of excess aqueous ammonia to a pink coloured aqueous solution...
Text Solution
|
- Match the complexes in Column I with their properties listed in Column...
Text Solution
|
- A, B and C are three complexes of chromium (III) with the empirical fo...
Text Solution
|
- Which of the following are all features of isomers of [Co (en)(3)] Cl(...
Text Solution
|
- What is the relationship between the following two complex ions ? (I...
Text Solution
|
- Which of the following complexes have doubtful existence ?
Text Solution
|
- The paramagnetic complexes is (are)
Text Solution
|
- Complexes expected to be coloured in solution is/are
Text Solution
|
- In case of octahedral compex, if the e(g) orbitals (d(x^(2) - y^(2)) "...
Text Solution
|
- In case of octahedral compex, if the e(g) orbitals (d(x^(2) - y^(2)) "...
Text Solution
|
- In case of octahedral compex, if the e(g) orbitals (d(x^(2) - y^(2)) "...
Text Solution
|
- Assertion [Ti (H(2)O)(6)] Cl(4) is colourless while [Sc (H(2)O)(6)] Cl...
Text Solution
|
- Assertion [Ni (CO)(4)] has longer C-O bond length than the same in [C...
Text Solution
|
- Match the quantity of Column I with the quantity of Column II
Text Solution
|
- How many stereoisomers exist for the complex [Co (en)(2) ClNO(2)] Br ?
Text Solution
|
- The complex Ca(2) [M (CN)](6) has spin only magnetic moment 2.83 BM an...
Text Solution
|