Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT-NUCLEAR PHYSICS-All Questions
- A sample contains two isotopes -one stale and the other unstable. Numb...
Text Solution
|
- In a beam of neurons the particles are having a kinetic energy of 0.03...
Text Solution
|
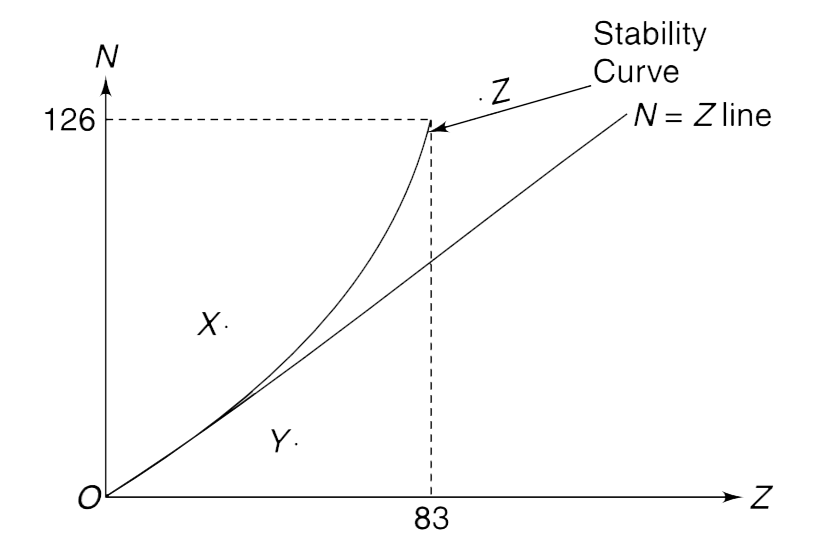
- The figure shows a plot of number of neutron (N) versus number of prot...
Text Solution
|
- Which of the following isotopes is more stable and why? (a) .(3)^(7)...
Text Solution
|
- Consider the position emission reaction .(z)^(A)Xrarrunderset(z-1)AY...
Text Solution
|
- Tritium (.(1)^(3)H) is a radioactive isotope of hydrogen. It has a hal...
Text Solution
|
- In the thorium decay series that begins with .^(232)Th,.^(228)Th alpha...
Text Solution
|
- Nuclei of .^(64)Cu can decay be electron capture (probability 61%) or ...
Text Solution
|
- In a sample there are two radioactive nuclide X and Y. Initially, popu...
Text Solution
|
- It is known that the ratio of .^(14)C to .^(12)C atoms in living being...
Text Solution
|
- Taking the data from last problem and assuming that 18% of our body ma...
Text Solution
|
- Consider the nuclear decay reaction. .(z)^(A)Xrarrunderset(z-1)AY+(+...
Text Solution
|
- The ratio (by weight) for U^(238) in a rock sample is 4:3. Assume that...
Text Solution
|
- A radioactive species has decay constant of lambda=10^(-2)s^(-1). Prob...
Text Solution
|
- A radioactive substance has a half life of t(0). Two particular nuclei...
Text Solution
|
- In a sample of radioactive material the probability that a particular ...
Text Solution
|
- Atomic mass of .(3)^(7)Li is 7.01600u and that of .(4)^(7)Be is 7.0169...
Text Solution
|
- .(15)P^(32) is beta active and has a short half life of 14 days. The ....
Text Solution
|
- A free neutron at rest, decays into three particles: a proton, an elec...
Text Solution
|
- A radioactive nucleus A decay to C after emitting two alpha and three ...
Text Solution
|