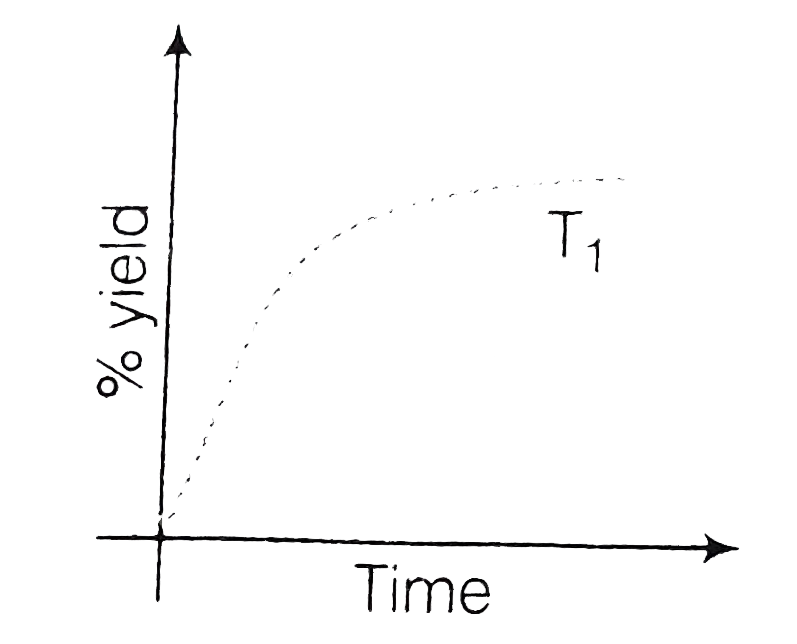
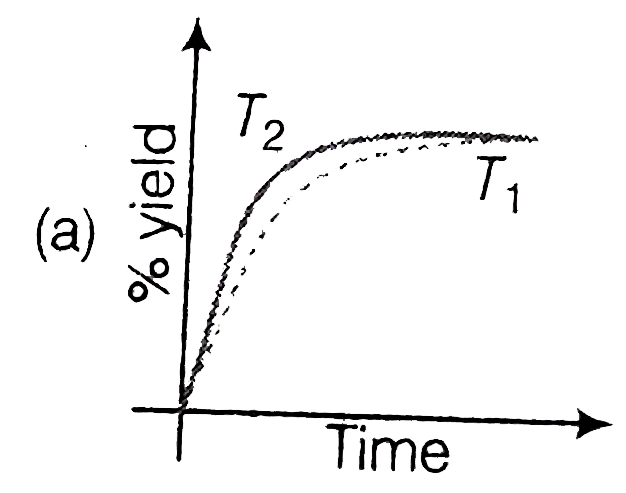
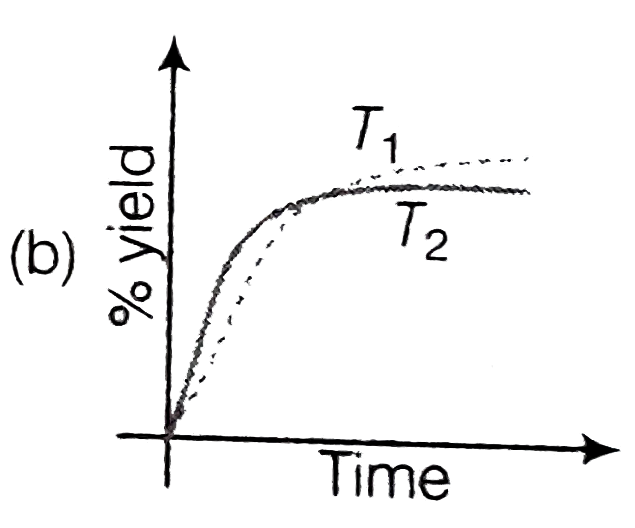
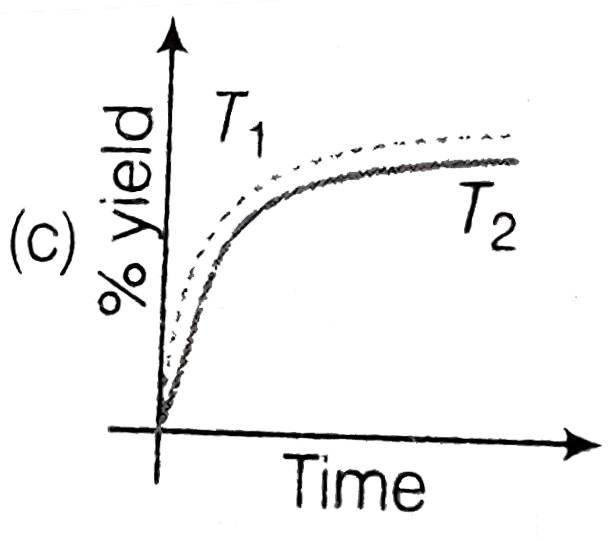
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL AND IONIC EQUILIBRIUM
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise OBJECTIVE_TYPE|3 VideosCHEMICAL AND IONIC EQUILIBRIUM
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise COMPREHENSION_TYPE|3 VideosCARBOXYLIC ACID AND THEIR DERIVATIVES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise CHAPTER TEST|3 VideosCHEMICAL BONDING
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise MATCH THE COLUMN|1 Videos
Similar Questions
Explore conceptually related problems