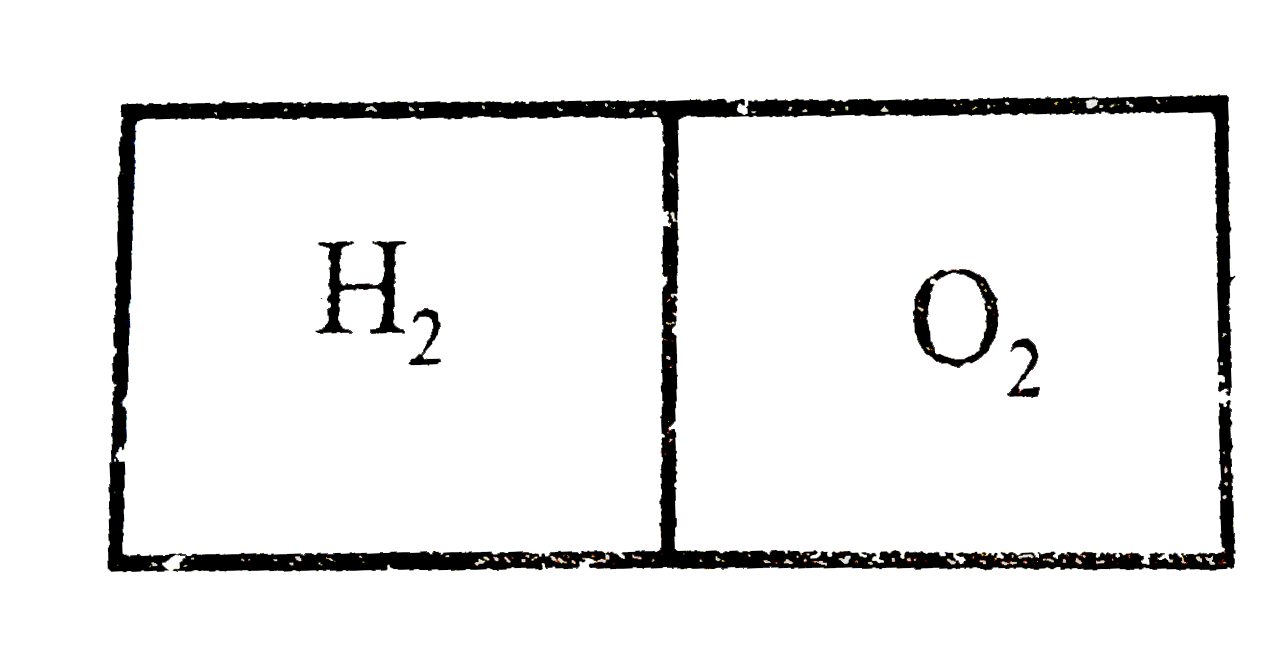Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-PHYSICS
- A particle starts from point A, moves along a straight line path with ...
Text Solution
|
- One end of a uniform rod of length 10m is placed in boiling water whil...
Text Solution
|
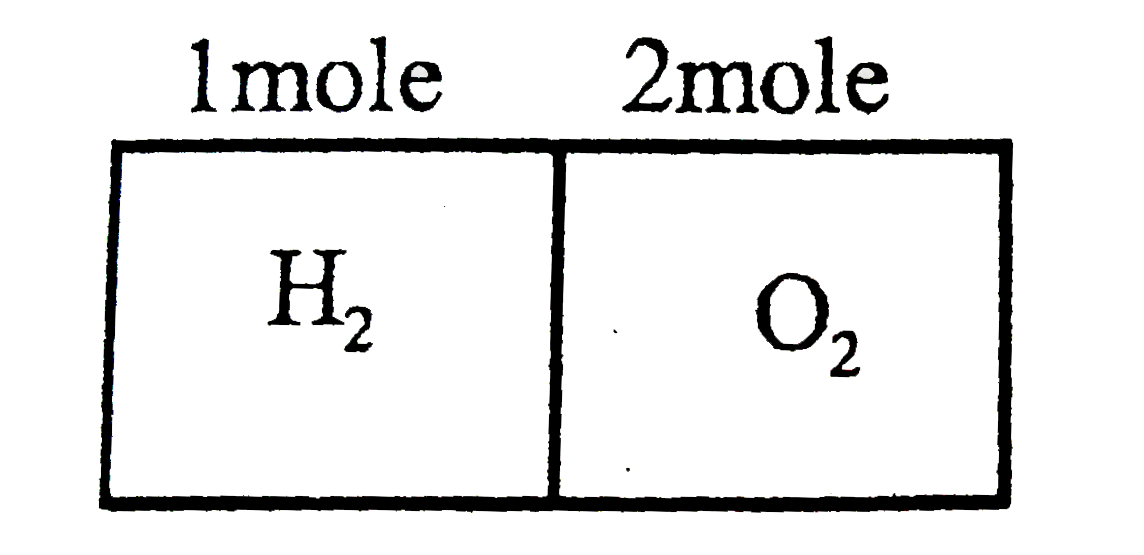
- 1 mole hydrogen and 2 moles oxygen are filled in neigboring parts of a...
Text Solution
|
- An iron ring of mass m1 oscillates in its an plane with time period of...
Text Solution
|
- In an adiabatic expansion the product of pressure and volume :
Text Solution
|
- A disc is performing pure rolling on a smooth stationary surface with ...
Text Solution
|
- If a wire is pulled by force of F keeping both end fixed, it oscillate...
Text Solution
|
- The angular amplitude of a simple pendulum is theta(0). The maximum te...
Text Solution
|
- If the ratio of lengths, radii and youngs's modulus of steel and and b...
Text Solution
|
- Two bodies of masses 2 kg and 3 kg are connected by a metal wire of cr...
Text Solution
|
- When a golf ball is hit by a club there are four phases to its motion ...
Text Solution
|
- When a golf ball is hit by a club there are four phases to its motion ...
Text Solution
|
- When a golf ball is hit by a club there are four phases to its motion ...
Text Solution
|
- A wave y= 3mm sin (2pix-200 pit) is Propagating in the left strin...
Text Solution
|
- A cube and a sphere (both solid) of same mass, same material are heate...
Text Solution
|
- Fig. shows a wave motion in which displacements of particles at an ins...
Text Solution
|
- An ideal gas undergoes an expansion from a state with temperature T(1)...
Text Solution
|
- Column -I containing equations of a particles different motions. Here ...
Text Solution
|
- A bob is attached to two strings of length 3m and 4 m as shown. The di...
Text Solution
|
- A glass bulb contains air and mercury. If the volume of air in bulb is...
Text Solution
|