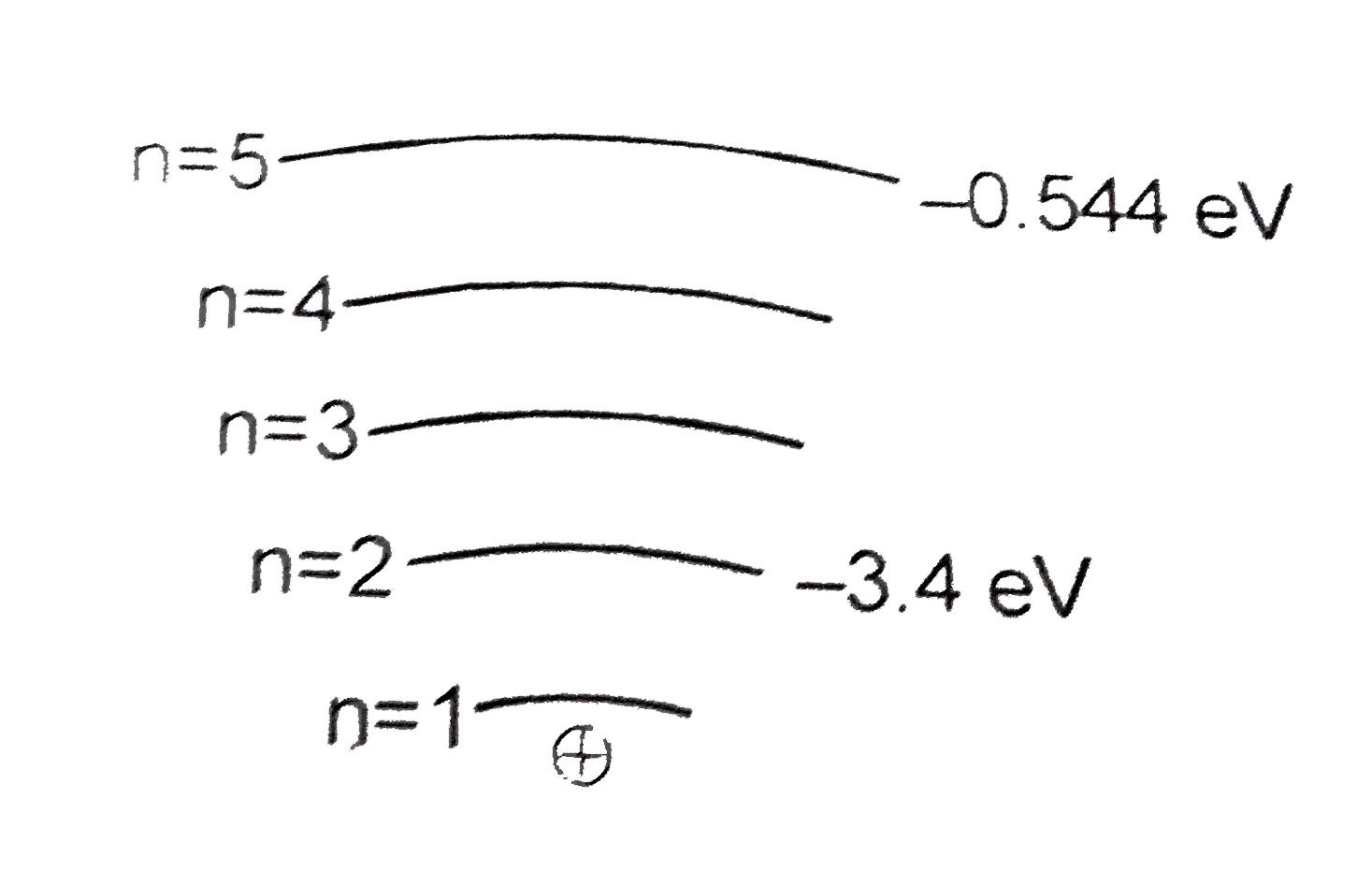A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VIBRANT-TEST PAPERS-PART - III : CHEMISTRY
- The appropriate reagent for the following transformation
Text Solution
|
- Which of the following metal sulphide liberate H(2)S gas on reaction w...
Text Solution
|
- Calculate EMF of following represented cell at 298 K Pb(s) |Pb^(2+)...
Text Solution
|
- The product B & C in above reaction respectively are :
Text Solution
|
- Ferric alum does not form precipitate with.
Text Solution
|
- The specific conductivity of N//10 KCl solution at 20^(@)C is 0.212 oh...
Text Solution
|
- Indicate the colour of compound Z formed in the given reaction , C(...
Text Solution
|
- The salt which does not form coloured metaborate in borax bead test.
Text Solution
|
- What is the amount of O(2) produced by electrolysis of water using a c...
Text Solution
|
- Which of the following will give Tollen's test (I) CH(3)-CH(2)-C-=C...
Text Solution
|
- If water pollution continues at its present rate, if will eventually
Text Solution
|
- Consider the following redox reaction occurring in acidic medium : ...
Text Solution
|
- Aunderset(Delta)overset(.^(-)OH)rarr1-"Acetyl-1-Cyclopentene". A wi...
Text Solution
|
- Biochemical Oxygen Demand, (BOD) is a measure of organic material pres...
Text Solution
|
- Calculate equivalent conductivity at infinite dilution of the salt KOO...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Number of moles of RMgX consumed during reaction are? , underset(2...
Text Solution
|
- A mixture of 0.1 mol of Na(2)O and 0.2 mol NaCl is dissolved in 1000g ...
Text Solution
|
- Which one of the following statement is incorrect ?
Text Solution
|
- Identify which product give lucas test with HCl+ZnCl(2)
Text Solution
|