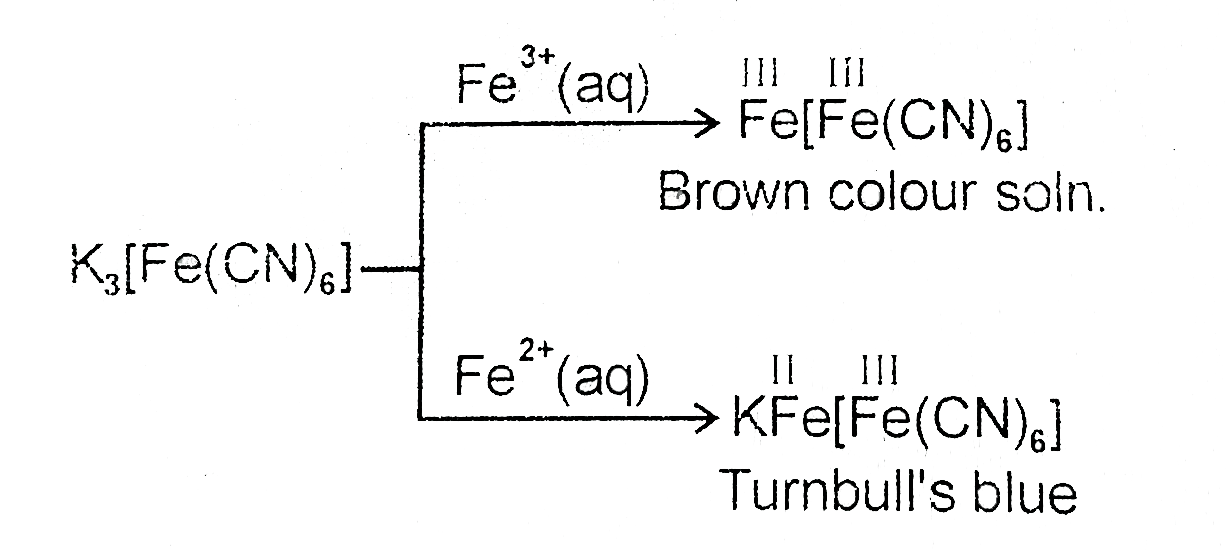A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VIBRANT-TEST PAPERS-PART - III : CHEMISTRY
- For which of the following enzyme catalysed reaction, maltase is the s...
Text Solution
|
- Which of the following regarding diborane is correct ? (I) It is a ...
Text Solution
|
- x = Number of structural isomer of C(4)H(10)O reacts with PhMgBr to ga...
Text Solution
|
- Under the influence of an electric field the particles in a sol migrat...
Text Solution
|
- Identify the incorrect statement(s) among the following : (I) Moist ...
Text Solution
|
- , overset(NaOBr)rarrP(1)underset(KOH)overset(CHCl(3))rarrP(2)underset(...
Text Solution
|
- In a solid (AB) having rock salt type structure all the atoms along bo...
Text Solution
|
- Select incorrect statement :
Text Solution
|
- Which of the given polymer is condensation polymer as well as Homopoly...
Text Solution
|
- Which is INCORRECT statement about the solution of H(2)O" and "C(2)H(5...
Text Solution
|
- The method of electrolytic refining is not suitable in the extraction ...
Text Solution
|
Text Solution
|
- Select the incorrect statement
Text Solution
|
- Magnesium is extracted by electrolysis of fused magnesium chloride usi...
Text Solution
|
- Identify the final product (Y) ,underset(Delta)overset((i)CHCl(3)/...
Text Solution
|
- which of the following is not correctly matched? {:("Dispersed phase"...
Text Solution
|
- In which of the following pair both minerals are oxide and metal is co...
Text Solution
|
- , underset((ii)H^(+)//Delta)overset((i)Br(2)//KOH)rarr Product is
Text Solution
|
- x g of glucose was dissolved in 500 g water and cooled upto -0.25^(@)C...
Text Solution
|
- Which of the following elements have low value of E^(Theta) (Electrode...
Text Solution
|