Similar Questions
Explore conceptually related problems
Recommended Questions
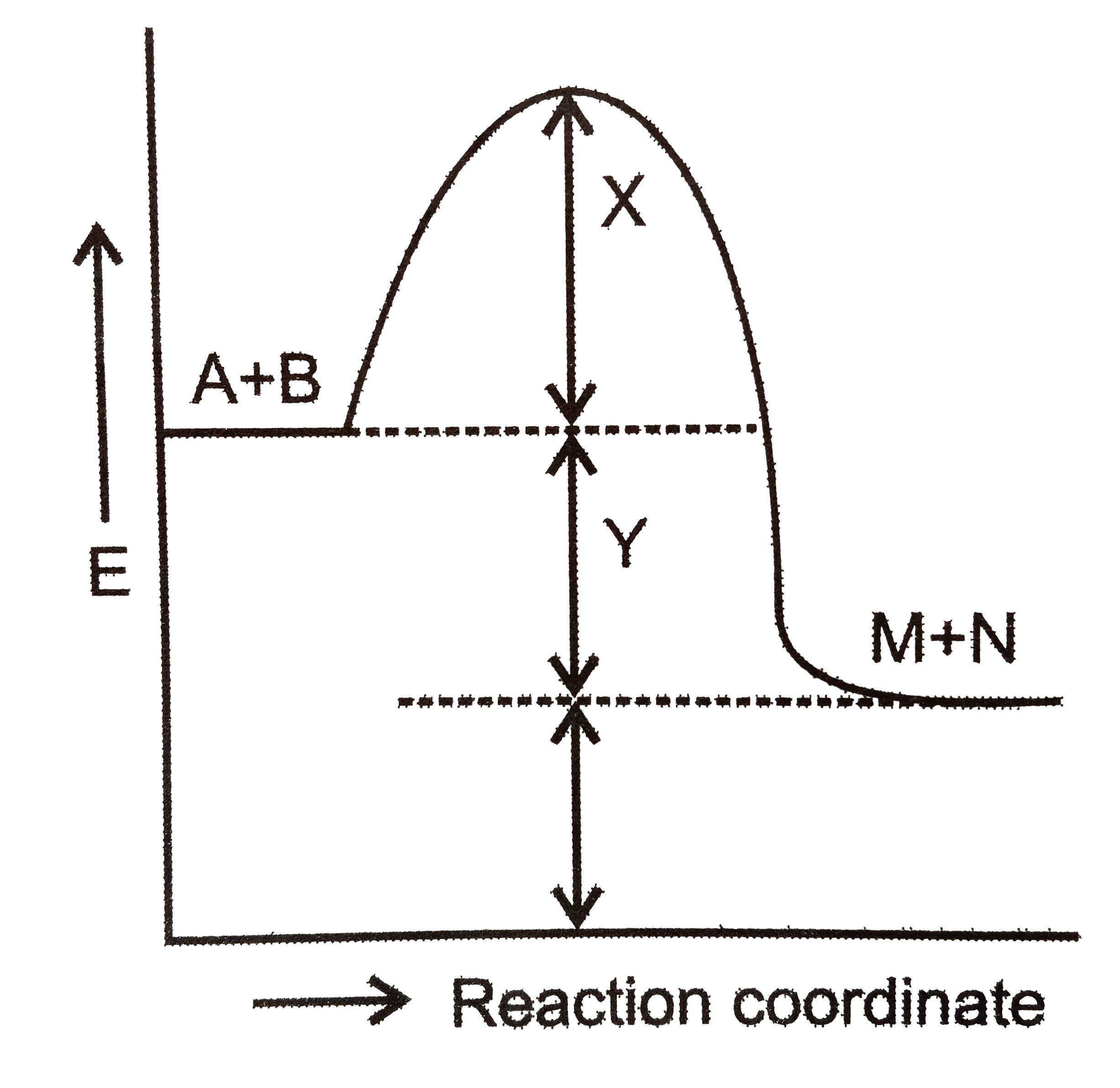
- Consider the following figure for the reaction: A+Brarr M+N Ans...
Text Solution
|
- Consider the following figure for the reaction: A+Brarr M+N Answer the...
Text Solution
|
- An endothermic reaction, Ararr B have an activation energy 15 kcal//mo...
Text Solution
|
- Energy of activation for a reversible reaction is 6 kcal (E(a) forward...
Text Solution
|
- Consider the endothermic reaction XrarrY with the activation energies...
Text Solution
|
- If the activation energy of forward reaction is four times the amount ...
Text Solution
|
- From the given graph, arrange the following in an ascending order : 1....
Text Solution
|
- For a reaction A + B hArr C+D , if the activation energy of backward ...
Text Solution
|
- Consider an endothermic reaction X to Y with the activation energies E...
Text Solution
|