A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-MASTER PRACTICE PROBLEM-Multiple objective
- Figure represents on the log-log scale pressure (P) dependence of adia...
Text Solution
|
- Two moles of O(2)(gamma=7/5) at temperature T(0) and 3 moles of CO(2)(...
Text Solution
|
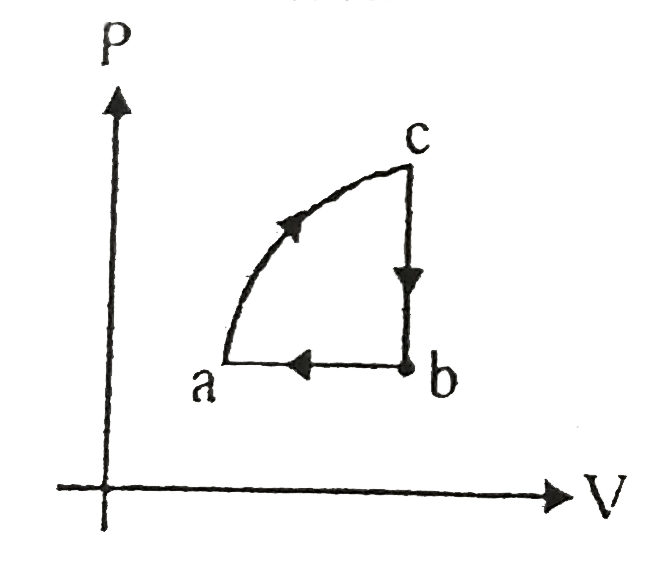
- In the given cyclic process from c to b 40 J heat is exchanged from b ...
Text Solution
|
- An insulating cylinder contains equal volumes of He and O(2) separated...
Text Solution
|
- The following figure-I .427 shows a block of mass m suspended from a f...
Text Solution
|
- Two large thin conducting plates with a small gap in between are place...
Text Solution
|
- A fixed point charge Q is at origin At t=0 a charge q with m is at x=a...
Text Solution
|
- How does the total energy stored in the capacitors in the circuit show...
Text Solution
|
- In the circuit shown, some potential difference is applied between A a...
Text Solution
|
- How does the total energy stored in the capacitors in the circuit show...
Text Solution
|
- Figure shows an arrangement of four identical ractangular plates A, B ...
Text Solution
|
- For the given circuit, select the correct alternative(s)
Text Solution
|
- A capacitor of capacity C(0) is conneted to a battery of emfV(0) When ...
Text Solution
|
- A dielectric slab fills the space between the plates of a parallel-pla...
Text Solution
|
- A galvanometer has a resistance of 96Omega and full scale deflection o...
Text Solution
|
- When a galvanometer is shunted with a 4Omega resistance the deflection...
Text Solution
|
- In the circuit shown, which of the following statements is correct?
Text Solution
|
- In the circnit shown, capacitor is initially uncharged till the switch...
Text Solution
|
- In the circuit shown there is steady state with the switch closed. The...
Text Solution
|
- In the circuit shown R(1)=R(2)=10Omega and resistance per unit length ...
Text Solution
|