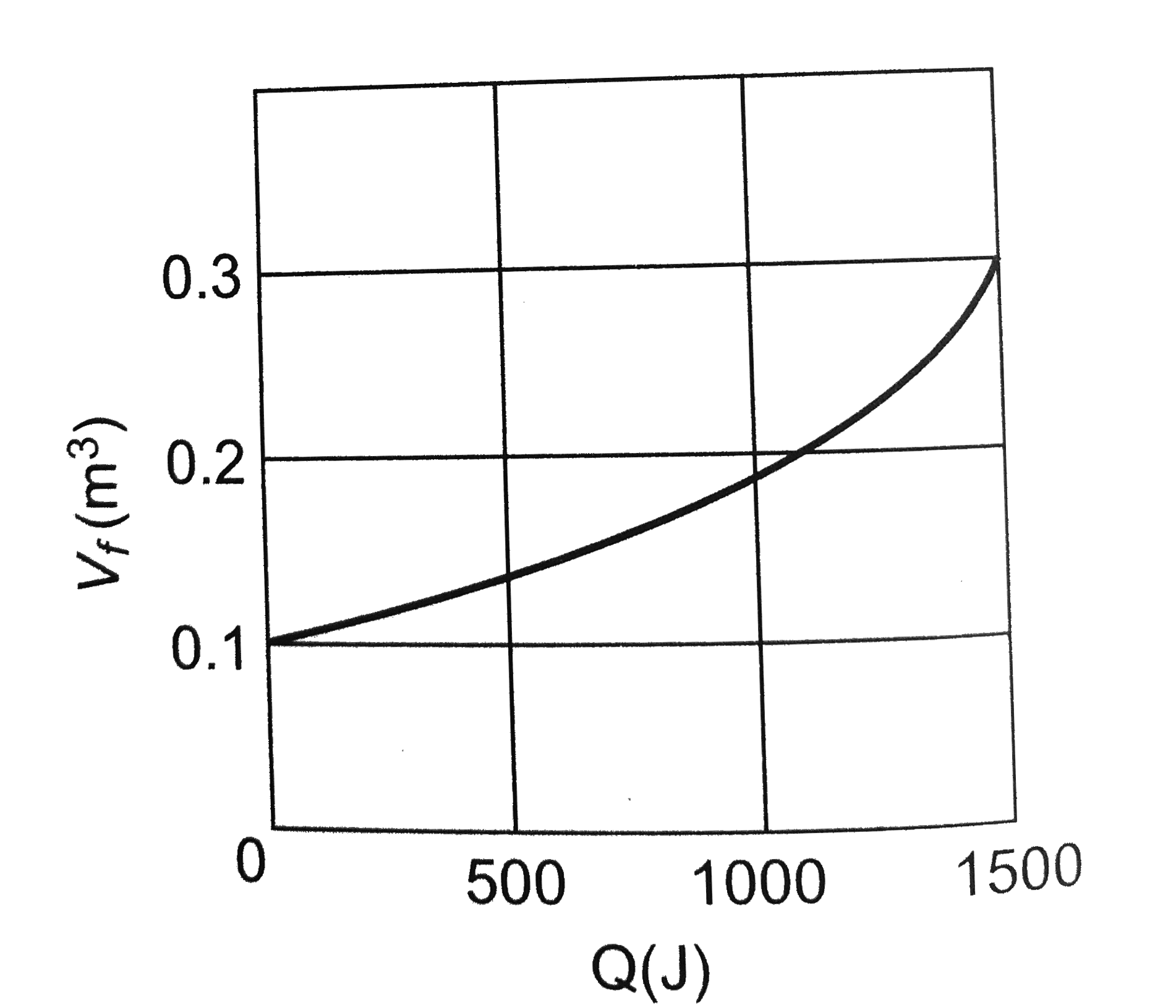
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-GMP ASSESMENT-Objective problems
- A non conducting rod AB of length sqrt(3)R, uniformly distributed char...
Text Solution
|
- The entire network shown here, has uniform wire having resistance per ...
Text Solution
|
- A block of mass m=5kg is placed on the wedge of mass M=32kg as shown i...
Text Solution
|
- A small collar of mass m is given an intial velocity of magnitude v(0...
Text Solution
|
- An ideal gas undergoes a circular cycle as shown in the figure. Find t...
Text Solution
|
- A particle is projected from point P on inclined plane OA perpendicula...
Text Solution
|
- A square plate of mass 10kg and side 20m is moving along the groove wi...
Text Solution
|
- A particle A is moving in xy plane with the constant speed of 2pi m//s...
Text Solution
|
- Three capacitors (of capacitances C, 2C and 3C) and three resistors (o...
Text Solution
|
- Three point masses m, 2m and m, connected with ideal spring (of spring...
Text Solution
|
- Two capillary tubes of diameters 3.0 mm and 6.0 mm are joined together...
Text Solution
|
- In the figure shown, two identical blocks A and B of mass m=1//2 kg ea...
Text Solution
|
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- A block of wood is floating in water such that 1//2 of it is submerge...
Text Solution
|
- Monochromatic radiation of wave length 640.2 nm (1nm=10^(-9)m) from a ...
Text Solution
|
- According to the Rydberg formula, the wavelengths of the first two spe...
Text Solution
|
- A bar of mass m is suspended horizontally on two vertical springs of s...
Text Solution
|
- One mole of an ideal monoatomic gas under goes a process V=aT^(2). The...
Text Solution
|
- The atomic mass number of Radium is A=226, its half life is 1622 years...
Text Solution
|
- A conducting movable rod AB lies across the frictionless parallel cond...
Text Solution
|