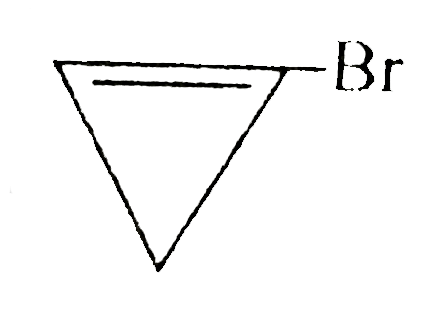
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-GENERAL ORGANIC CHEMISTRY-Exercise 2
- Compound A gives white precipitate with AgNO(3) and a highly stable ca...
Text Solution
|
- Compound A gives white precipitate with AgNO(3) and a highly stable ca...
Text Solution
|
- Compound A gives white precipitate with AgNO(3) and a highly stable ca...
Text Solution
|
- Statement-I: CH(3)-overset(O)overset(||)(C)-CH(2)-overset(O)overset(||...
Text Solution
|
- Statement-I: All C-C bod lengths are equal in CH(2)=CH-CH=CH(2) Sta...
Text Solution
|
- Statement-I: is an anti-aromatic compound. Statement-II: Cyclic, pl...
Text Solution
|
- Give the correct order of initials T or F for following statements Use...
Text Solution
|
- Choose the correct statement:
Text Solution
|
- Among the following which one is having conjugated system:
Text Solution
|
- Which of the following compound is /are aromatic:
Text Solution
|
- Select the correct statement about product A.
Text Solution
|
- In each set of species select the aromatic species.
Text Solution
|
- Identify electron-donating groups in resonance among the following.
Text Solution
|
- Which one of the following pairs of structures represents the phenomen...
Text Solution
|
- In which of the followig pairs II^(nd) compound has less resonance ene...
Text Solution
|
- In which of the following pairs II^(nd) compound has higher resonance ...
Text Solution
|
- Resonance energy will be more if
Text Solution
|
- Select the correct statement (s):
Text Solution
|
- A canonical structure will be more stable if
Text Solution
|
- In which of the following lone-pair indicated is not involved in reson...
Text Solution
|