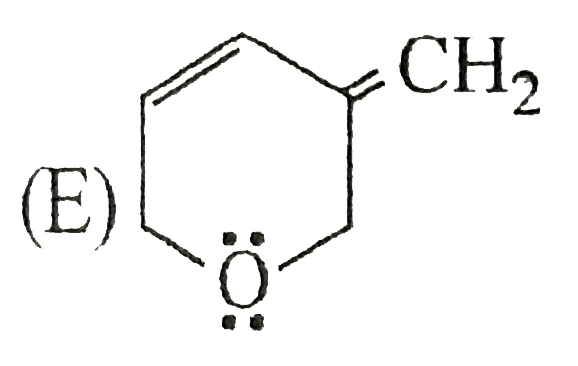
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-GENERAL ORGANIC CHEMISTRY-Exercise 2
- In which of the following pairs II^(nd) compound has higher resonance ...
Text Solution
|
- Resonance energy will be more if
Text Solution
|
- Select the correct statement (s):
Text Solution
|
- A canonical structure will be more stable if
Text Solution
|
- In which of the following lone-pair indicated is not involved in reson...
Text Solution
|
- In which of the following lone-pair indicated is involved in resonance...
Text Solution
|
- Which of the following statement is/are correct?
Text Solution
|
- Which of the following statement(s) is/are true.
Text Solution
|
- Select stable molecule(s)
Text Solution
|
- Which of the following is/are aromatic species.
Text Solution
|
- Which of the following reaction will form stable ion.
Text Solution
|
- Which of the following statements are incorrect
Text Solution
|
- In which of the following pairs II is more stable than I
Text Solution
|
- Which of the following is/are correct order of resonance energy in giv...
Text Solution
|
- Select true statement(s):
Text Solution
|
- Select the correct options
Text Solution
|
- Which of the following orders are correct?
Text Solution
|
- Which of the following reaction equilibrium is favoured in forward dir...
Text Solution
|
- Match the column
Text Solution
|
- Match the names of carboxylic acids in column I with pk(a) value in co...
Text Solution
|