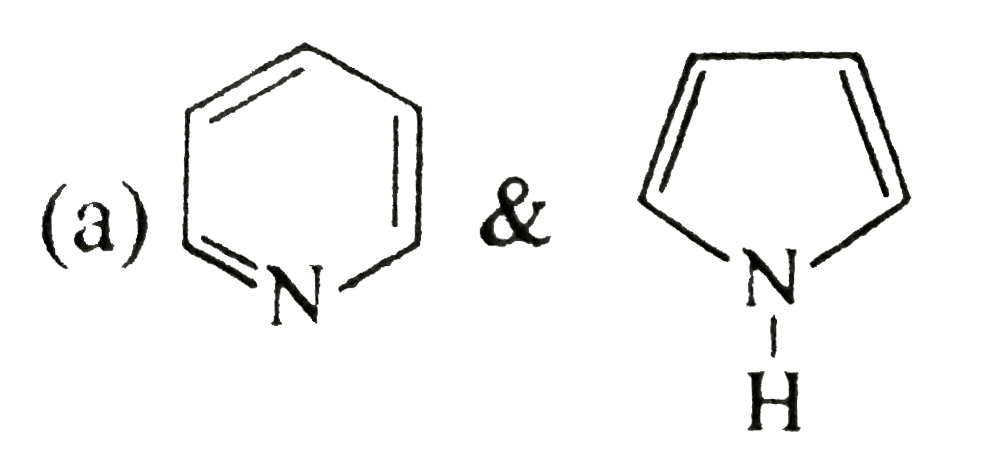Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-GENERAL ORGANIC CHEMISTRY-Exercise 3
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write Stability order of following intermediates:
Text Solution
|
- Rank the following sets of intermediates in increasing order of their ...
Text Solution
|
- In each of the following pairs of ions which ion is more stable:
Text Solution
|
- Rank the following sets of intermediates in increasing order of their ...
Text Solution
|
- Rank the following sets of intermediates in increasing order of their ...
Text Solution
|
- Compare heat of hydrogenation (Decreasing order)
Text Solution
|
- Write increasing order of heat of hydrogenation:
Text Solution
|
- In which of the following pairs, indicated bond is of greater strength...
Text Solution
|
- Consider the given reaction: In the above reaction which one of t...
Text Solution
|
- Write correct order of acidic strength of following compounds: (i). ...
Text Solution
|
- Write increasing order of basic strength of following: (i). (a) F^(ɵ...
Text Solution
|
- Record the following sets of compounds according to increasing pK(a)(=...
Text Solution
|
- Explain which is a stronger acid CH(3)CH(3)&BrCH(2)NO(2)
Text Solution
|
- Which of the following pairs has higher resonance energy: (a). CH(3)...
Text Solution
|
- Choose the more stable alkene in each of the following pair. Explain y...
Text Solution
|
- Which of the followig pairs has higher resonance energy: (a). (b)...
Text Solution
|
- Rank the following sets of intermediates in increasing order of their ...
Text Solution
|