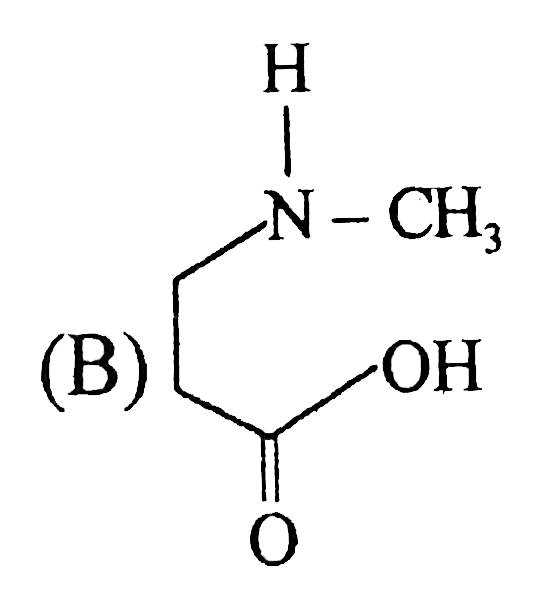
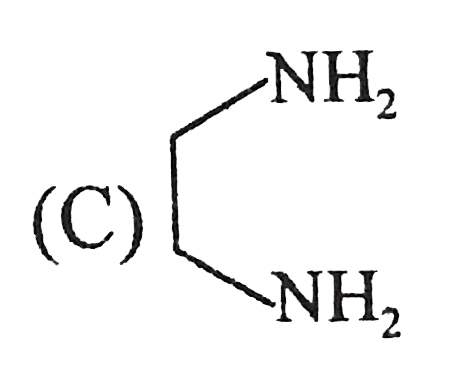
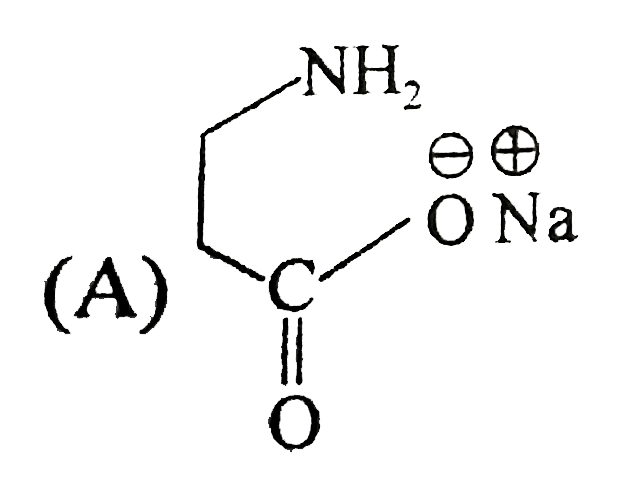A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE-2 Reasoning Type|3 VideosNITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE-2 Multiple Correct Choice Type|17 VideosNITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE 1|30 VideosGENERAL ORGANIC CHEMISTRY
BANSAL|Exercise Exercise 3|19 VideosP BLOCK ELEMENTS
BANSAL|Exercise Exercise 3|20 Videos
Similar Questions
Explore conceptually related problems
BANSAL-NITROGEN COMPOUNDS-EXERCISE-2 PARAGRAPH
- The conversion of an amide by action of NaOH and Br(2) to primary amin...
Text Solution
|
- The conversion of an amide by action of NaOH and Br(2) to primary amin...
Text Solution
|
- The conversion of an amide by action of NaOH and Br(2) to primary amin...
Text Solution
|
- Ketoxine when heated with certain reagents undergoes rearrangement to ...
Text Solution
|
- Ketoxine when heated with certain reagents undergoes rearrangement to ...
Text Solution
|
- Ketoxine when heated with certain reagents undergoes rearrangement to ...
Text Solution
|
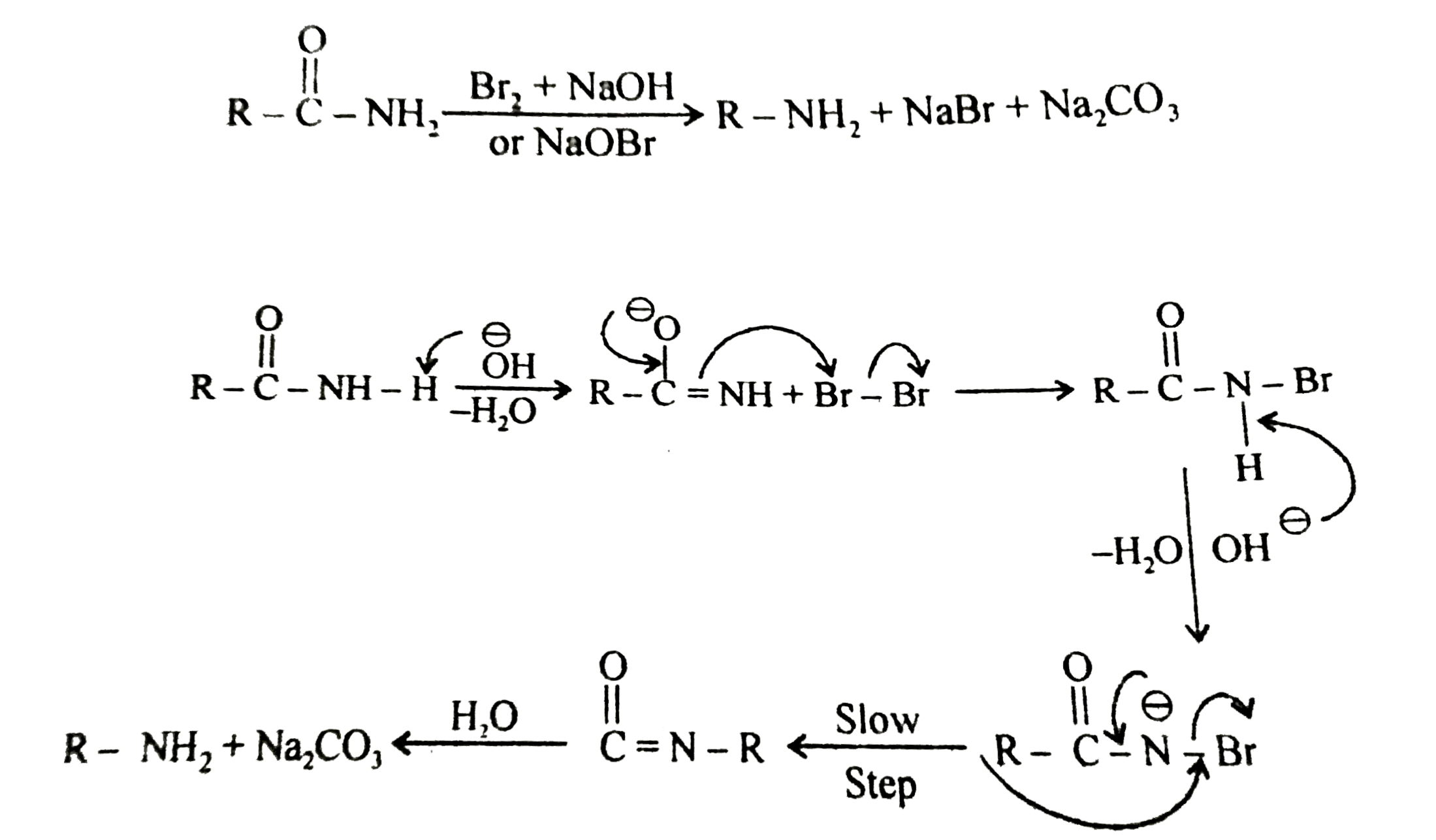
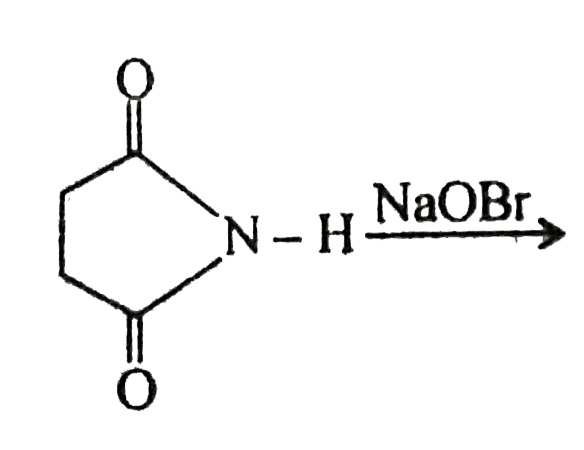 , Find out X :
, Find out X :