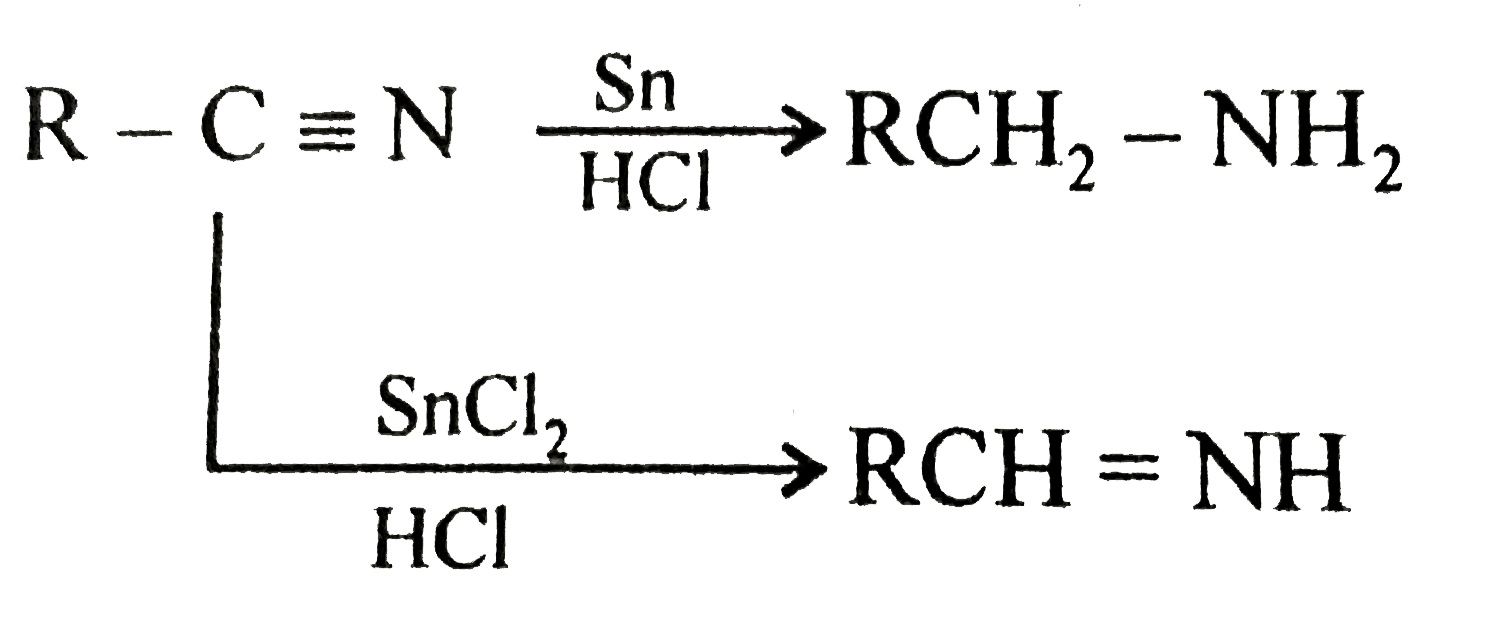
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE-2 Multiple Correct Choice Type|17 VideosNITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE- 2 MATCH THE COLUMN|3 VideosNITROGEN COMPOUNDS
BANSAL|Exercise EXERCISE-2 PARAGRAPH|6 VideosGENERAL ORGANIC CHEMISTRY
BANSAL|Exercise Exercise 3|19 VideosP BLOCK ELEMENTS
BANSAL|Exercise Exercise 3|20 Videos
Similar Questions
Explore conceptually related problems