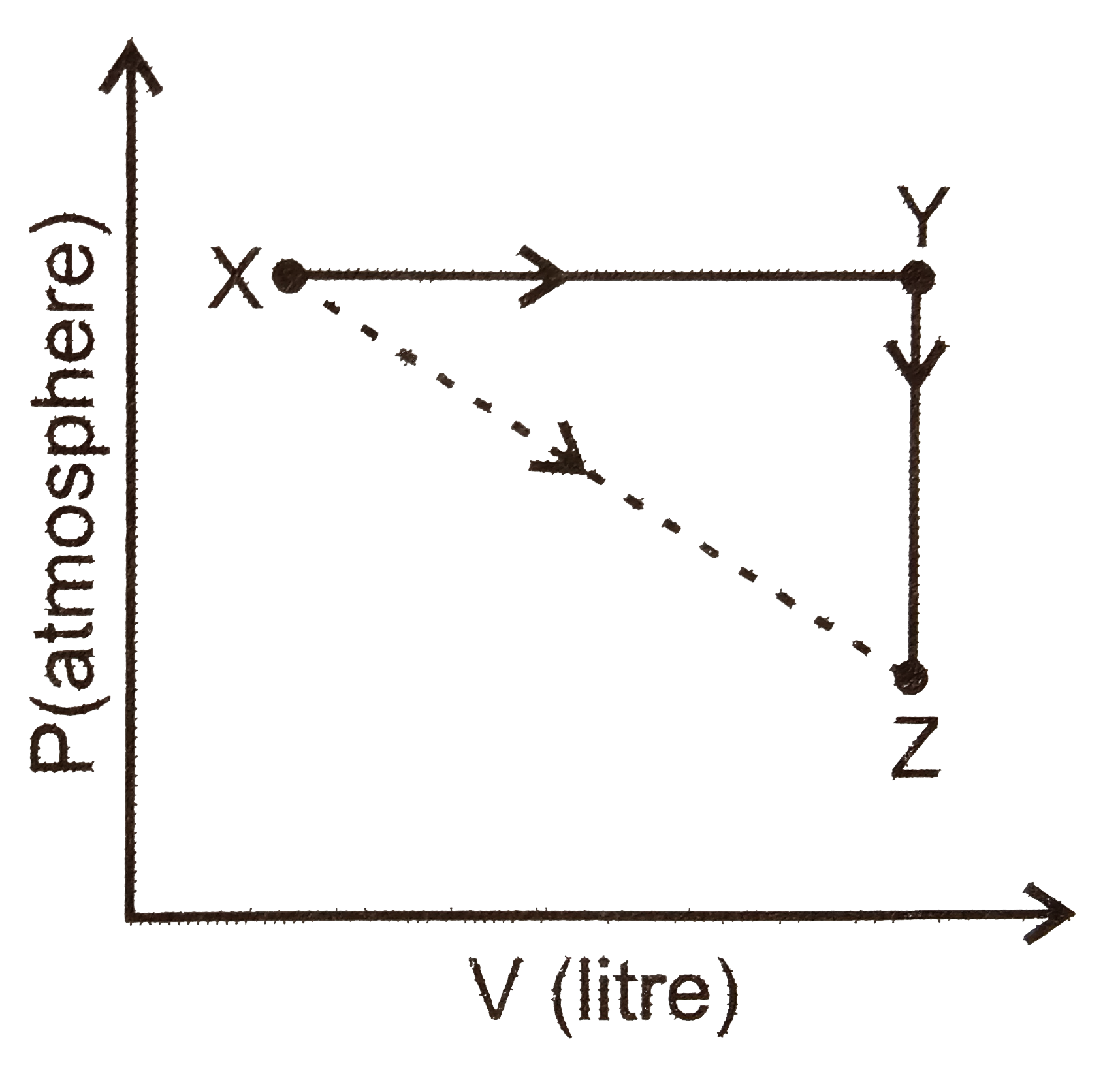
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-THERMODYNAMICS AND THERMOCHEMISTRY-JEE Main And Advanced
- Benzene and naphthalene form an ideal solution at room temperature. Fo...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- Among the following, the intensive property is (properties are) :
Text Solution
|
- Among the following , the state funcation (s) is (are)
Text Solution
|
- Identify the extensive quantities from the following (i) refractive ...
Text Solution
|
- Assertion (A) : There is a natural asymmetry between converting work t...
Text Solution
|
- Assertion (A): For every chemical reaction at equilibrium, standard Gi...
Text Solution
|
- Assertion: The heat absorbed during the isothermal expansion of an ide...
Text Solution
|
- A fixed mass m of a gas is subjected to transfromation of states from ...
Text Solution
|
- A fixed mass m of a gas is subjected to transformation of state: K to ...
Text Solution
|
- Match the thermodynamic processes given under column I with the expres...
Text Solution
|
- Match the transformations In column I with appropriate options in colu...
Text Solution
|
- Enthalpy is an ……………….. property.
Text Solution
|
- When Fe(S) is dissovled in aqueous hydrochloric acid in a closed vesse...
Text Solution
|
- The heat content of the products is more than that of the reactants in...
Text Solution
|
- A system is said to be………..if it can neither exchange matter nor energ...
Text Solution
|
- C(P) -C(V) for an ideal gas is………….. .
Text Solution
|
- The total energy of 1mol of an ideal monatomic gas at 27^(@)C is……. .
Text Solution
|
- The first law of thermodynamics is not adequate in predicting the dire...
Text Solution
|