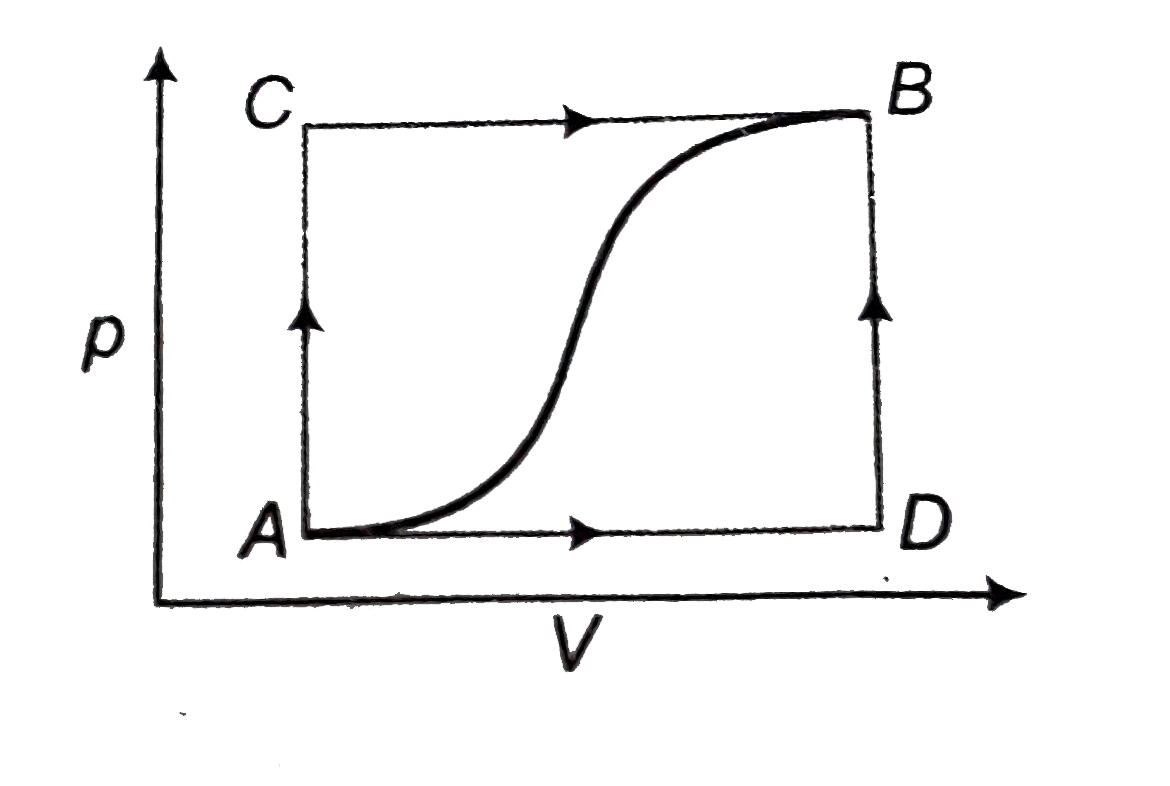
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-THERMODYNAMICS AND THERMOCHEMISTRY-JEE Main And Advanced
- The polymerisation of ethylene to linear polyethylene is represented b...
Text Solution
|
- In order to get maximum calorific output a burner should have an optim...
Text Solution
|
- Determine enthalpy change for, C(3)H(8(g))+H(2(g))rarr C(2)H(6(g))+C...
Text Solution
|
- Using the data ( all vaues in kcal mol^(-1) at 25^(@)C) given below, c...
Text Solution
|
- The enthalpy of combustion of H(2) , cyclohexene (C(6)H(10)) and cyclo...
Text Solution
|
- An intimate mixture of ferric oxide and aluminium is used as solid fue...
Text Solution
|
- The standard molar heats of formation of ethane, carbon dioxide, and l...
Text Solution
|
- The bond dissociation energy of gaseous H(2),Cl(2) and HCl are 104,58 ...
Text Solution
|
- Given the following standard heats of reactions: (a) heat of formati...
Text Solution
|
- Given that: i. C(s) + O(2)(g) rarr CO(2)(g) , DeltaH =- 94.05 kcal ...
Text Solution
|
- The standared enthalpies of formation at 298K for C C1(g), H(2)O(g), C...
Text Solution
|
- The enthalpies for the following reactions (DeltaH^(Theta)) at 25^(@)C...
Text Solution
|
- the standed reaction free energy for N(2)O(4)(g) to 2NO(2)is DeltaG...
Text Solution
|
- we consider a grwing plant. Limting our system to plant itself, to be ...
Text Solution
|
- One mole of a monationc ideal gas intially at a pressure of 2.00 bar a...
Text Solution
|
- How much heat flows into the system along path ADB it the work done by...
Text Solution
|
- When the system is retuned form state B to A along the curved path, th...
Text Solution
|
- Assertion All reversible reaction proceeds in direction of lower Gibbs...
Text Solution
|
- Assertion lsothemal expansion of ideal gas againt vacuum must be simul...
Text Solution
|
- Match the statements of column I with values of column II.
Text Solution
|