Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-CHEMICAL KINETICS-JEE Main And Advanced
- An organic compound undergoes first decompoistion. The time taken for ...
Text Solution
|
- 2X(g) rarr 3Y(g)+2Z(g) {:("Time (in min)",0,100,200,):} |{:("Parti...
Text Solution
|
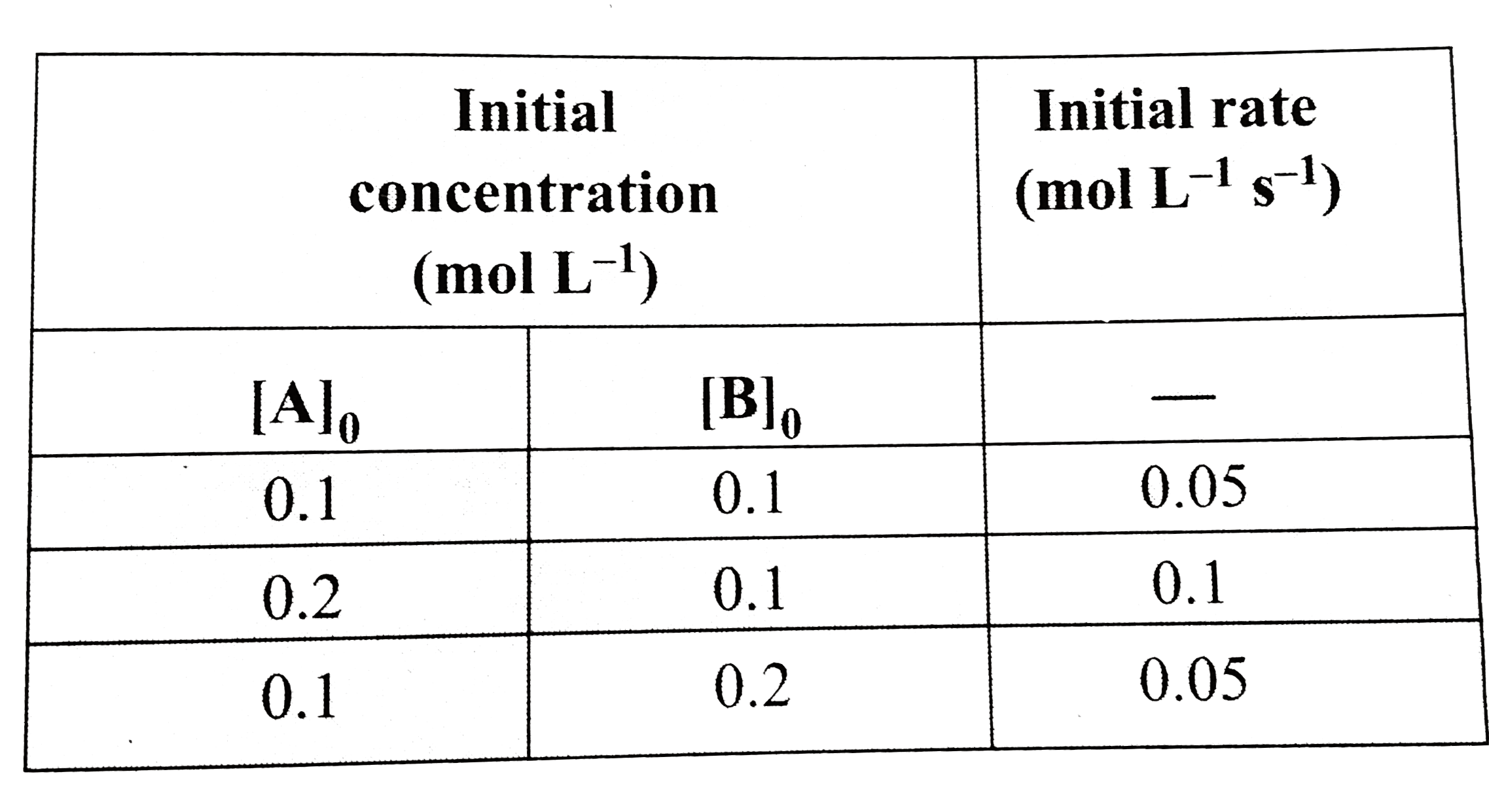
- For the given reaction A+B rarr Products, the following data are g...
Text Solution
|
- The rate of first order reaction is 0.04 mol L^(-1) s^(-1) at 10 min a...
Text Solution
|
- A hydrogenation reaction is carried out at 500 K. If the same reaction...
Text Solution
|
- The rate constant for an isomerization reaction, A rarr B is 4.5 xx 10...
Text Solution
|
- the rate constant of a reaction is 1.5xx10^(7) s^(-1) at 50^(@)C and ...
Text Solution
|
- The rate constant for the first order decompoistion of a certain react...
Text Solution
|
- One of the hazards of nuclear explosion is the generation of .^(90)Sr ...
Text Solution
|
- At 380^(@)C , the half-life periof for the first order decompoistion o...
Text Solution
|
- form the following data for the reaction between A and B, (a) Cal...
Text Solution
|
- The gas phase decomposition of dimethyl ether follows first order kine...
Text Solution
|
- A first order reaction A rarr B requires activation energy of 70 kJ mo...
Text Solution
|
- Two reaction, (I)A rarr Products and (II) B rarr Products, follow firs...
Text Solution
|
- The nuclide ratio, .(1)^(3) H to .(1)^(1) H in a sample of water is 8....
Text Solution
|
- The decompoistion of N(2)O(5) according to the equation 2N(2)O(5)(g) r...
Text Solution
|
- In a Arrhenius equation for a certain reaction, the values of A and E(...
Text Solution
|
- AN experiment requires minimum beta activity produced at the rate of 3...
Text Solution
|
- A first order gas reaction has k = 1.5 xx 10^(-6) s^(-1) at 200^(@)C. ...
Text Solution
|
- While studying the decompoistion of gaseous N(2)O(5), it is observed t...
Text Solution
|