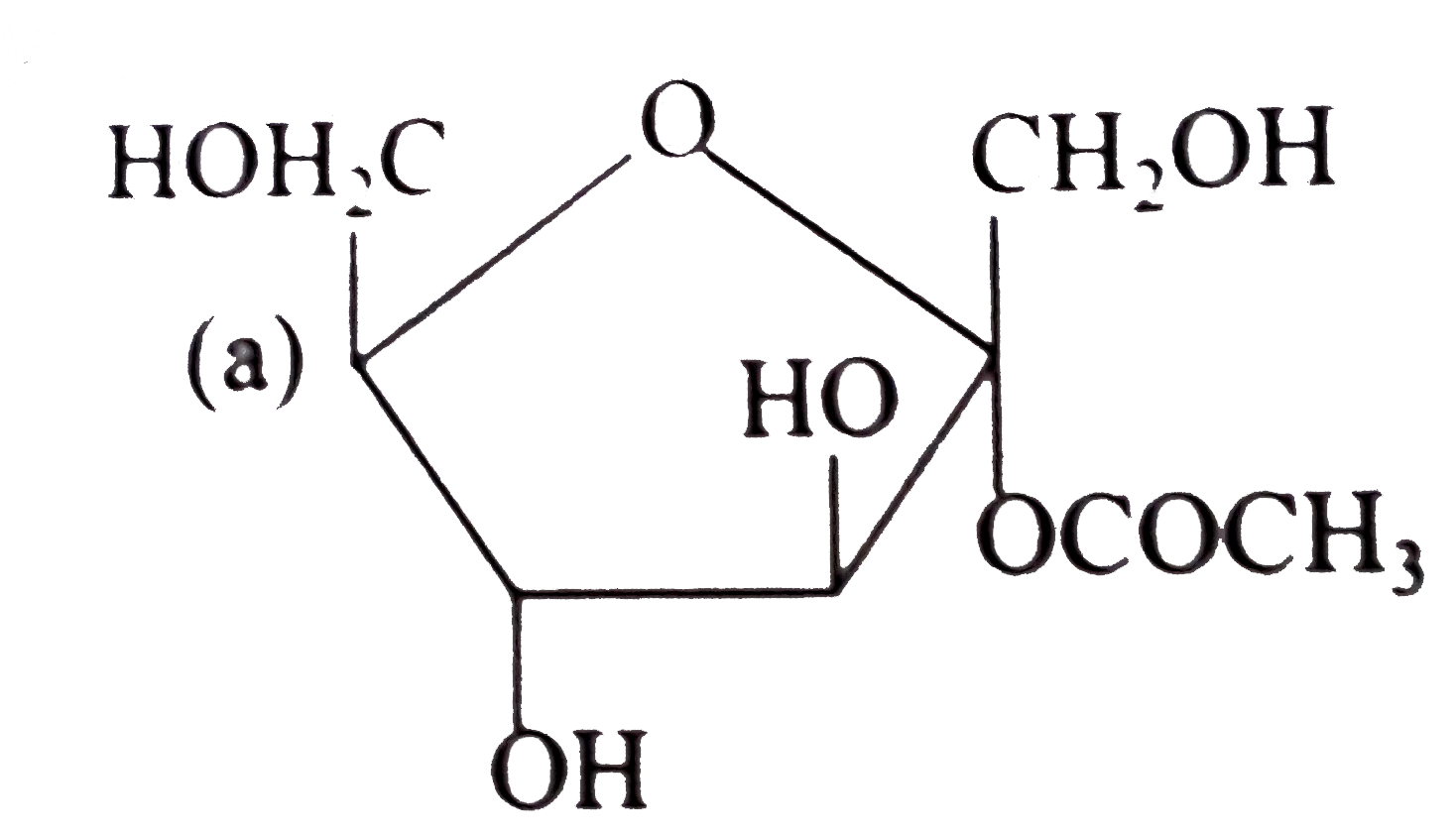
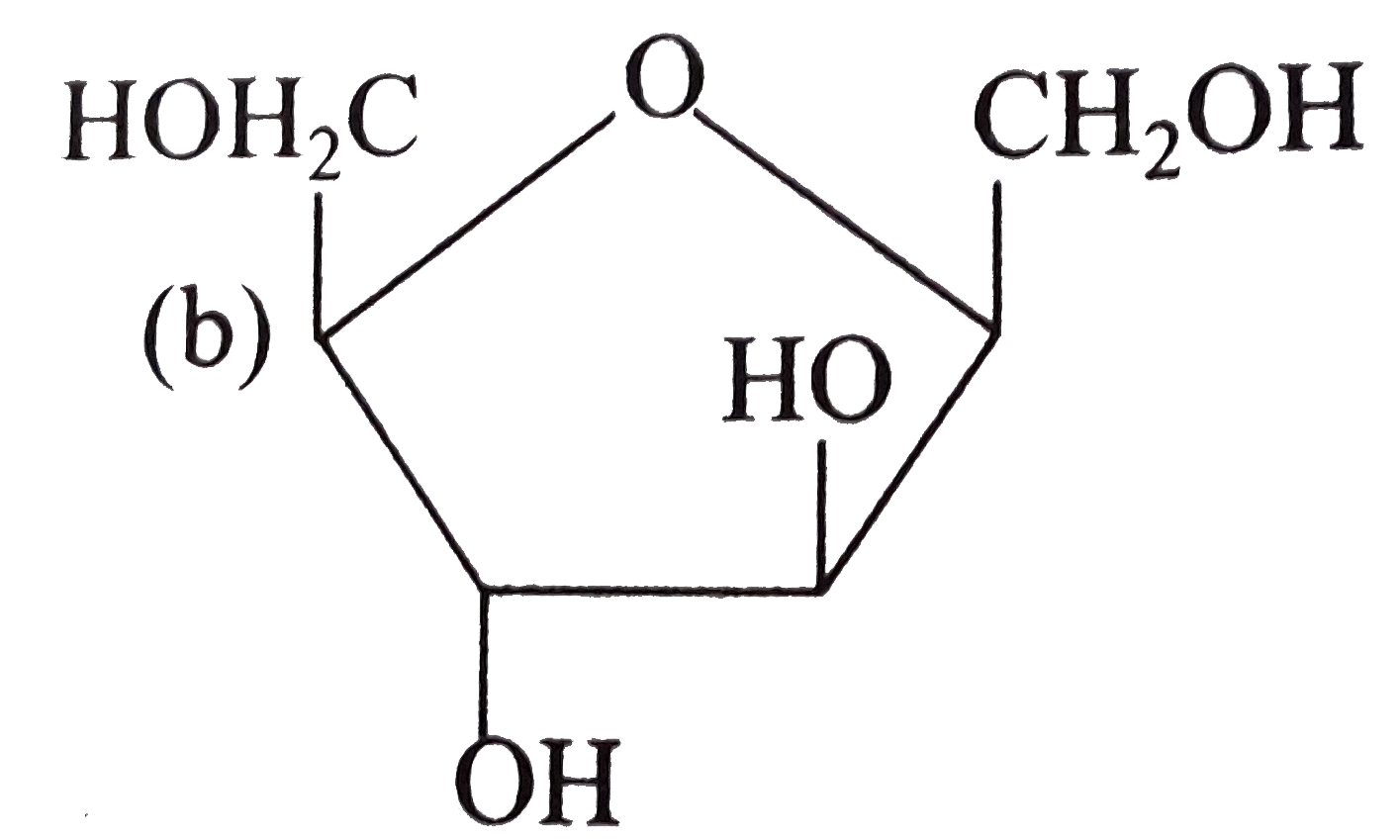
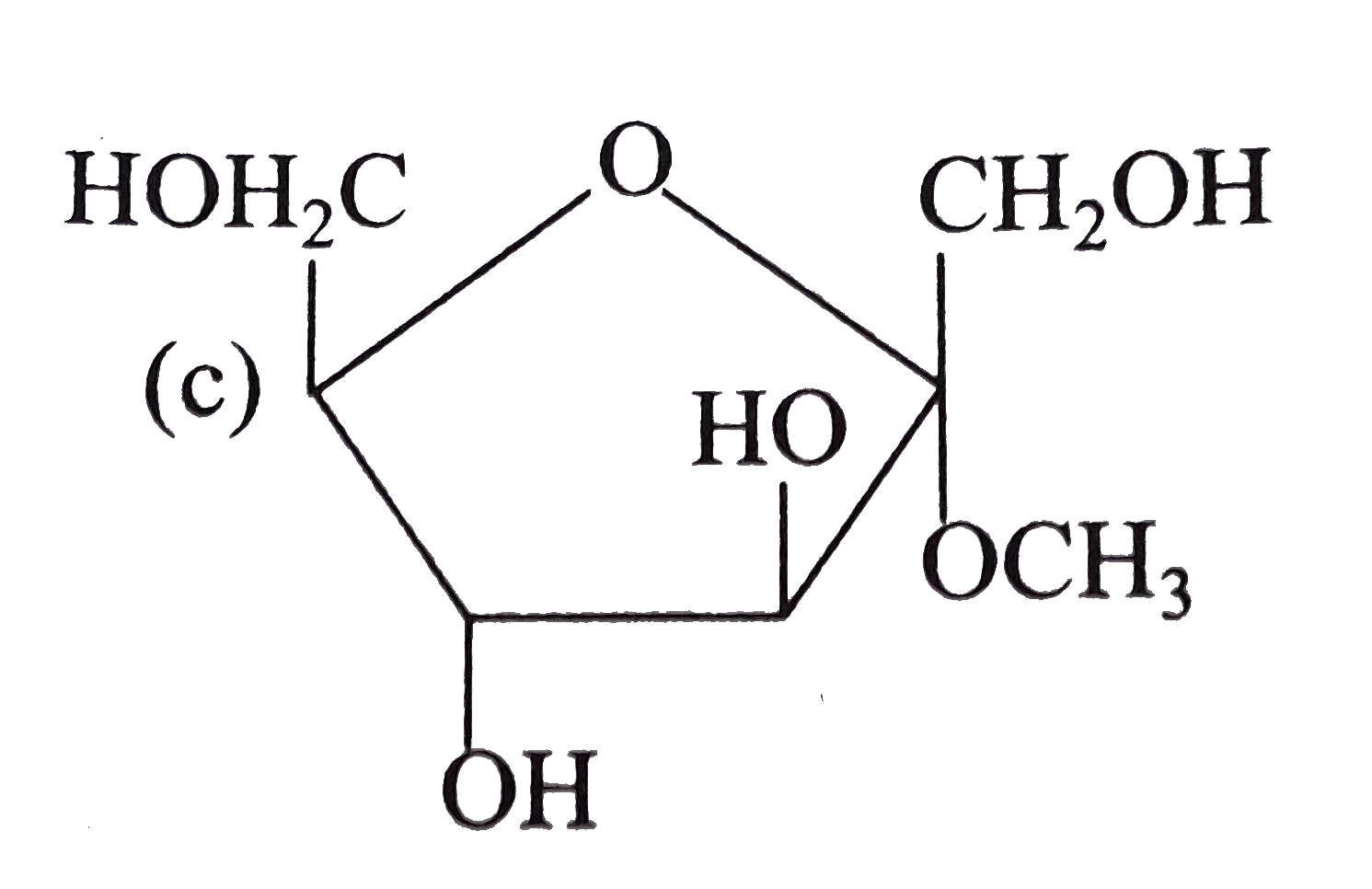
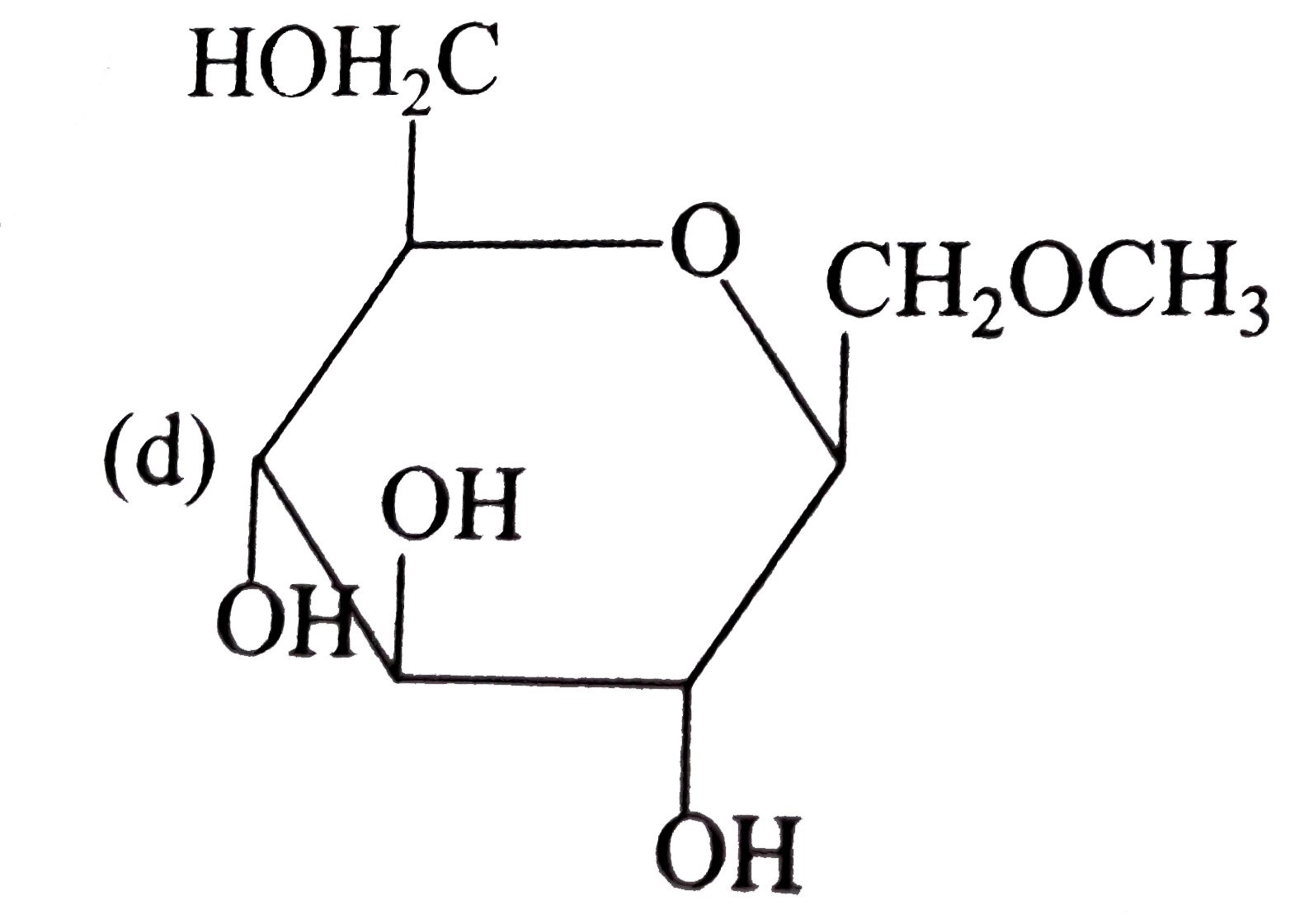
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES AND CHEMISTRY IN DAILY LIFE
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Chapter Test|9 VideosBENZENE AND ALKYL BENZENE
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise JEE Main And Advanced|56 VideosCARBOXYLIC ACID AND THEIR DERIVATIVES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise CHAPTER TEST|3 Videos
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-BIOMOLECULES AND CHEMISTRY IN DAILY LIFE-Chapter Test
- Which of the following compounds will behave as a reducing sugar in an...
Text Solution
|
- Which Fischer structure represents the open chain form of the followin...
Text Solution
|
- Which of the following compounds does not undergo mutarotation ?
Text Solution
|
- Which of the following is/are characteristic of an alpha-amino acid at...
Text Solution
|
- In which of the following case, the forms of amino acid and pH is (are...
Text Solution
|
- Compound A is a D-aldopentose that on oxidation with dilute NHO(3) giv...
Text Solution
|
- Compound A is a D-aldopentose that on oxidation with dilute NHO(3) giv...
Text Solution
|
- Compound A is a D-aldopentose that on oxidation with dilute NHO(3) giv...
Text Solution
|
- Assertion (A) D-glucose when dissolved in water undergo mutarotation w...
Text Solution
|
- Match the quantity of Column I with the quantity of Column II {:(,"C...
Text Solution
|