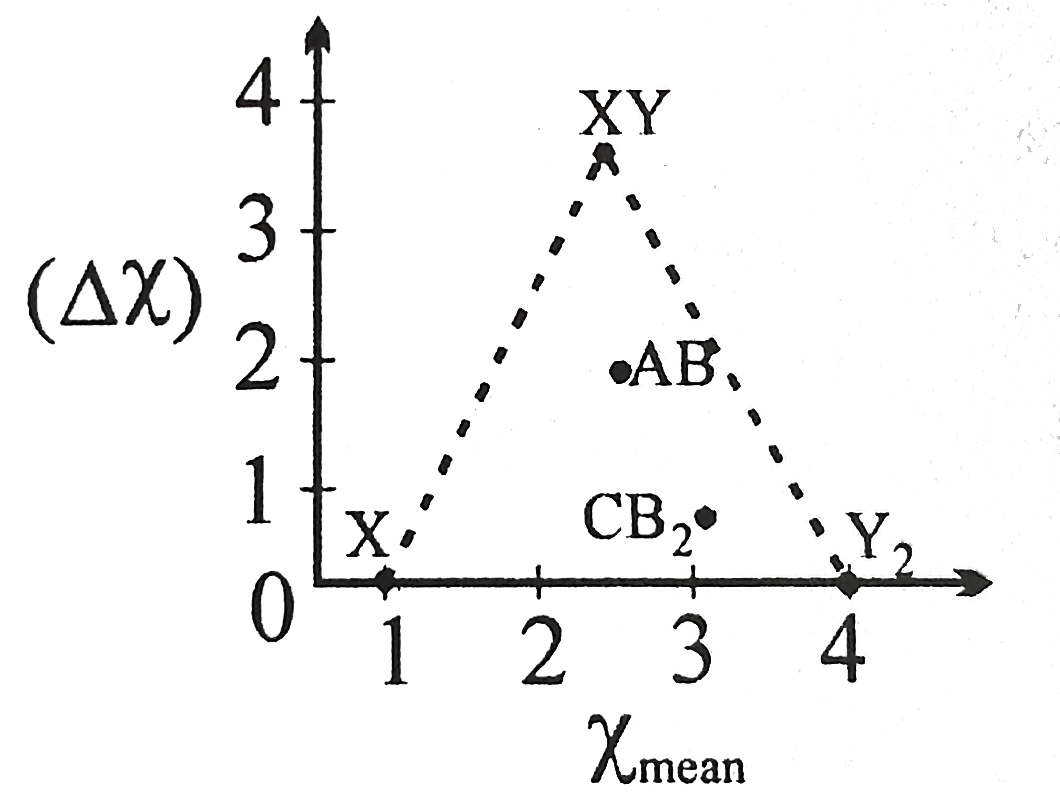A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-PERIODIC TABLE-EXERCISE-1
- Which of the following factors may be regarded as the main cause the l...
Text Solution
|
- The size of the species, Pb,Pb^(2+), Pb^(4+) decreases as -
Text Solution
|
- A Ketelaar triangle showing a plot of average electrongativity (chi(me...
Text Solution
|
- Match list-I with list-II and select the correct answer using the code...
Text Solution
|
- The actinoids exhibit more number of oxidation states in general than ...
Text Solution
|
- For the process X(g) +e^(-) rarr X^(-)(g), DeltaH = x and X^(-)(g)...
Text Solution
|
- Sodium forms Na^(+) ion but it does not form Na^(+2) because -
Text Solution
|
- Lanthanoids are-
Text Solution
|
- Bond distance C-F in (CF(4)) & Si-F in (SiF(4)) are respective 1.33 Å...
Text Solution
|
- The lowest electronegativity of the element from the following atomic ...
Text Solution
|
- The correct order regarding the electronegativity of hybrid orbitals o...
Text Solution
|
- If there were 10 periods in the periodic table then how many elements ...
Text Solution
|
- The correct order of increasing hydration energy of following ion is
Text Solution
|
- Consider the M(OH)(3) formed by all the group 13 elements. The correct...
Text Solution
|
- (a),(b) and (c) are elements in the second short period. Oxide of (a) ...
Text Solution
|
- Set containng isolectronic species is-
Text Solution
|
- Mendeleev's periodic table is based on atomic masses of elements. It w...
Text Solution
|
- If the ionic radii of each K^(+) and F^(-) are 1.34Å, then tha atomic ...
Text Solution
|
- The As-CI bond distance in AsCI(3) is 2.20 Å. Estimate the SBCR (singl...
Text Solution
|
- The IE values of Al((g)) = Al^(+) +e is 577.5 kJ mol^(-) and DeltaH fo...
Text Solution
|