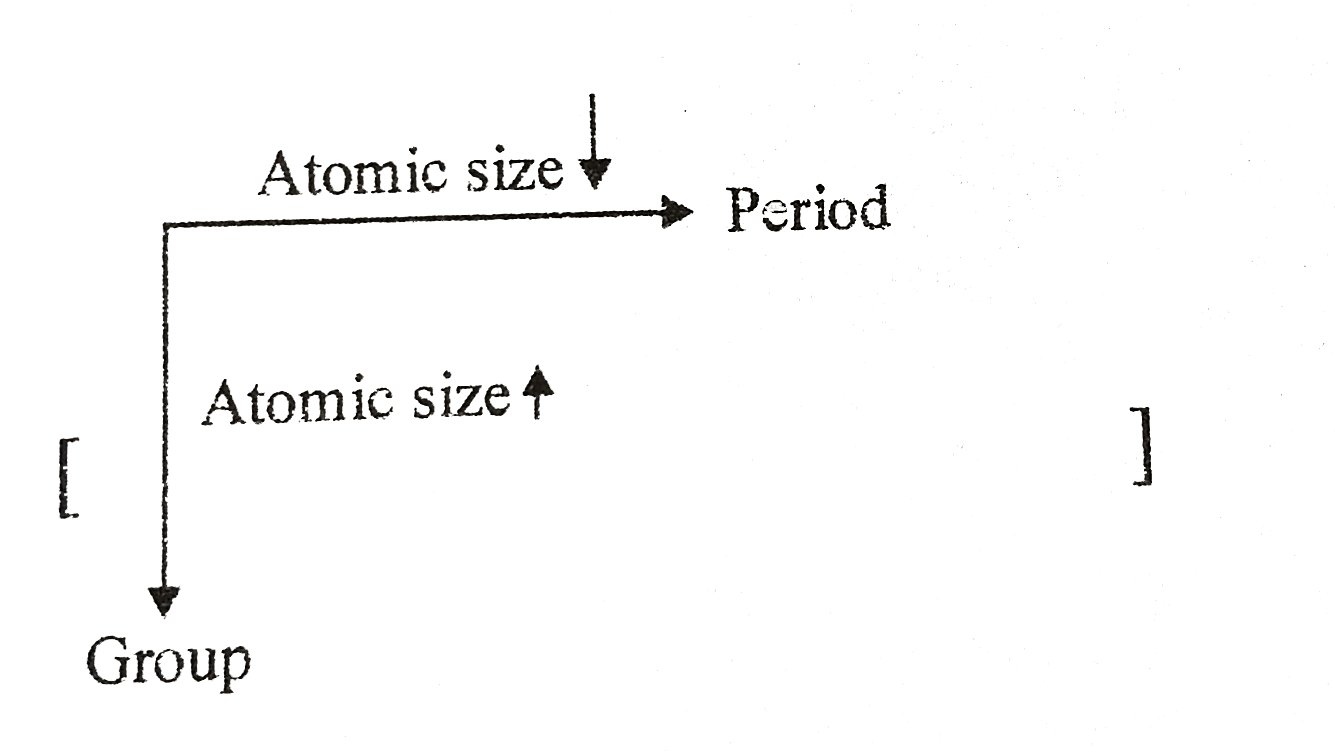Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-PERIODIC TABLE-EXERCISE-3
- Mg^(2+), O^(2-),Na^(+),F^(-),N^(3-) (Arrange in decreasing order of io...
Text Solution
|
- Why Ca^(2+) has a smaller ionic radius than K^(+).
Text Solution
|
- Arrange in decreasing order of atomic size: Na,Cs, Mg, Si, CI
Text Solution
|
- Why the first ionisation energy of carbon atom is greater than that of...
Text Solution
|
- The IE do not follow a regular trend in II & III periods with increasi...
Text Solution
|
- Explain why a few elements such as Be(+0.6),N(+0.3) & He(+0.6) have po...
Text Solution
|
- Which bond in each pair is more polar (a) P-CI or P - Br (b) S -CI o...
Text Solution
|
- From among the elements,choose the following: CI,Br, F,Al, C,Li,Cs& Xe...
Text Solution
|
- In the ionic ompound KF, the K^(+) and F^(-) ions are found to have pa...
Text Solution
|
- Which oxide is more basic, MgO or BaO? Whay?
Text Solution
|
- The basic nature of hydroxides of group 13 (III-A) decreases progressi...
Text Solution
|
- Based on location in P.T. which of the following would you expect to b...
Text Solution
|
- Compare the following giving reasons Acidic nature of oxides: CaO, CO,...
Text Solution
|
- The ionisation potentials of atoms A and B are 400 and 300 kcal mol^(-...
Text Solution
|
- If internuclear distance between CI atoms in CI(2) is 10Å & between H ...
Text Solution
|
- The As-CI bond distance in AsCI(3) is 2.20 Å. Estimate the SBCR (singl...
Text Solution
|
- The Pt-CI distance is 2.32 A in several crystaline compounds What is...
Text Solution
|
- The IE values of Al((g)) = Al^(+) +e is 577.5 kJ mol^(-) and DeltaH fo...
Text Solution
|
- For the gaseous reaction K+F rarrK^(o+)+F^(ɵ) Delta H = 19 kcal mo...
Text Solution
|
- Calculate E.N. of chlorine atom on Pauling scale if I.E. of CI^(-) is ...
Text Solution
|