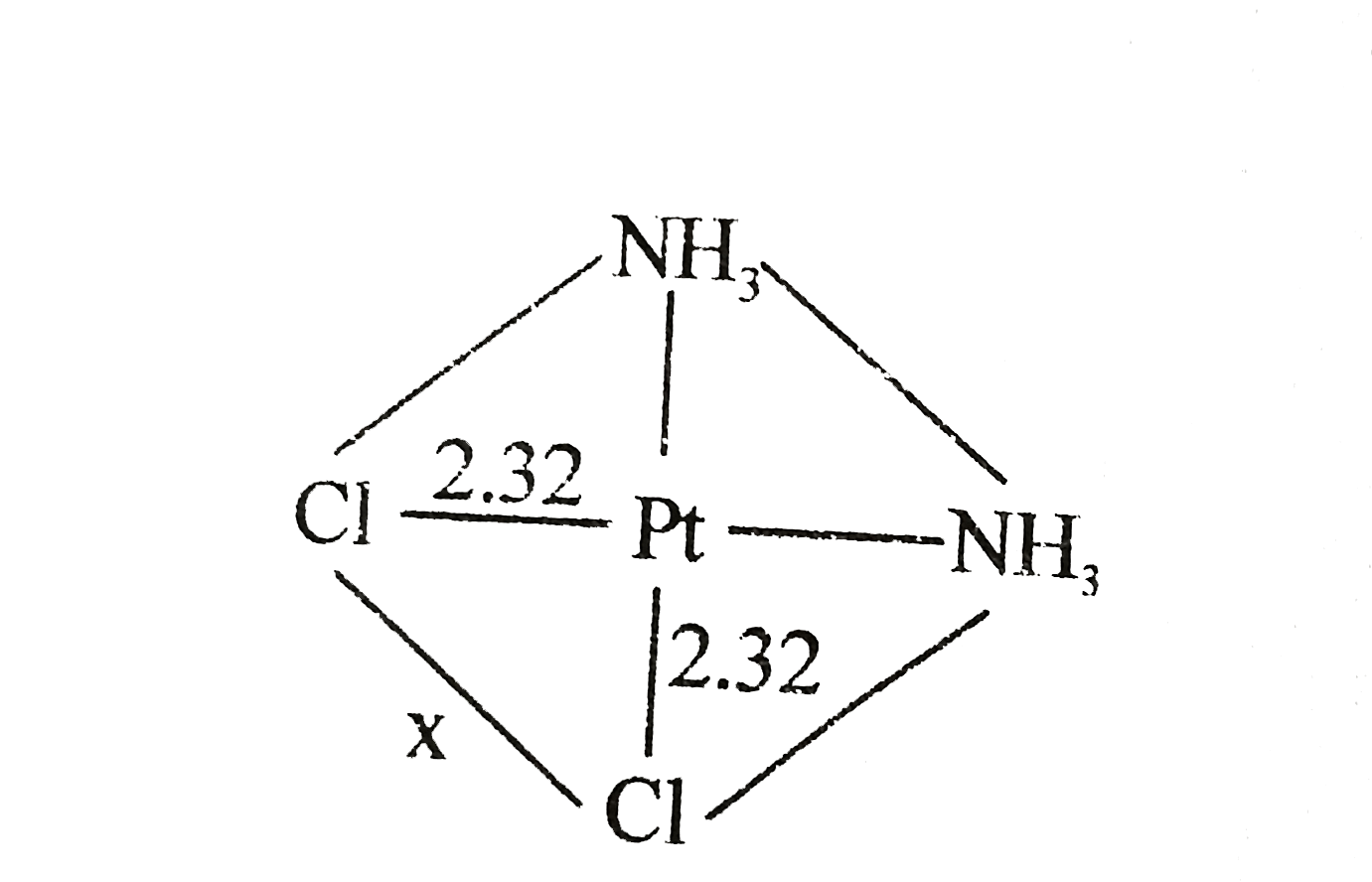Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-PERIODIC TABLE-EXERCISE-3
- Compare the following giving reasons Acidic nature of oxides: CaO, CO,...
Text Solution
|
- The ionisation potentials of atoms A and B are 400 and 300 kcal mol^(-...
Text Solution
|
- If internuclear distance between CI atoms in CI(2) is 10Å & between H ...
Text Solution
|
- The As-CI bond distance in AsCI(3) is 2.20 Å. Estimate the SBCR (singl...
Text Solution
|
- The Pt-CI distance is 2.32 A in several crystaline compounds What is...
Text Solution
|
- The IE values of Al((g)) = Al^(+) +e is 577.5 kJ mol^(-) and DeltaH fo...
Text Solution
|
- For the gaseous reaction K+F rarrK^(o+)+F^(ɵ) Delta H = 19 kcal mo...
Text Solution
|
- Calculate E.N. of chlorine atom on Pauling scale if I.E. of CI^(-) is ...
Text Solution
|
- Calculate the electronegativity of fluorine from the following data: ...
Text Solution
|
- Calculate the E.N. of CI from the bond energy of CIF (61 K Cal//"mol")...
Text Solution
|
- How many chlorine atoms will be ionised CI rarr CI^(+) +e^(-1) by the ...
Text Solution
|
- A mixture contains F and Cl atoms . The removal of an electron form e...
Text Solution
|
- Calculate the lattice energy of NaCI crystal from the following data b...
Text Solution
|
- Calculate the electron affinity of iodine with the help of the followi...
Text Solution
|
- The lattice enthalpy of solid NaCI is 772 kJ mol^(-1) and enthalpy of ...
Text Solution
|
- From the following information {:(A^(-)(g)rarrA^(+2)(g)+3e^(-),Delta...
Text Solution
|
- For ionic compound A^(2+) 2B^(-) & 2C^(+)D^(2-) (a) A(4)(S) +B(2)(g)...
Text Solution
|
- Prove that the formation of NaCI(2) is highly endothermic and energeti...
Text Solution
|
- Given: DeltaH(EG) of A^(+) =- 5x DeltaH(EG) of A^(2+) =- 8x DeltaH...
Text Solution
|
- What is the maximum number of electron possible in Ni^(+) having same ...
Text Solution
|
 .
.