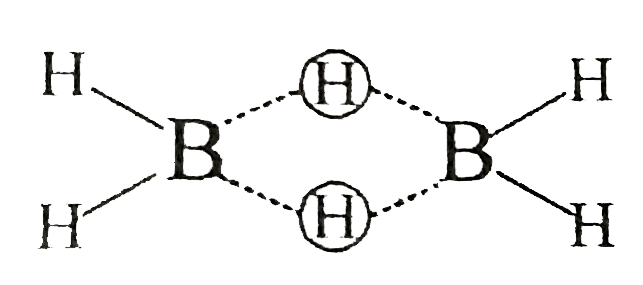
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-P BLOCK ELEMENTS-Exercise 3
- Which of the following statement is//are correct regarding graphite?
Text Solution
|
- {:(,"Column-I(Reactions)",,,"Column-II(Product)"),((A),"Borax",overset...
Text Solution
|
- {:(,"Column-I",,"Column-II"),((A),BBr(3)+H(2)rarrB,(p),"Borax bead tes...
Text Solution
|
- What is the nature aqueous solution of alums ?
Text Solution
|
- how are alums prepared?
Text Solution
|
- What are pseudo alums ?
Text Solution
|
- How can you prove that AlOH(3) is amphoteric in nature ?
Text Solution
|
- What is the nature of AlCl(3) in aqueous state ?
Text Solution
|
- Which allotropic of carbon is thermodynamically more stable ?
Text Solution
|
- What is tin disease, tin pest or tin plague ?
Text Solution
|
- What is tin cry ?
Text Solution
|
- What is the colour of C(60) in toluene ?
Text Solution
|
- What happens when calcium cynamide is hydrolysed ?
Text Solution
|
- What happens when K(4)Fe(CN)(6) is heated strongly ?
Text Solution
|
- What are the nature of CO(2),SiO(2),GeO(2),SnO(2),PbO(2) ?
Text Solution
|
- What happens when SiO(2) is reacted with following ? (a) KOH, (b) Ca...
Text Solution
|
- What is silica garden ?
Text Solution
|
- SiF(6)^(2-) exist but not CF(6)^(2-) explain why ?
Text Solution
|
- Find the maximum number of atoms are lying in the same plane for B(2)H...
Text Solution
|
- Which of the following substances is having higher lattice energy than...
Text Solution
|