Text Solution
Verified by Experts
Topper's Solved these Questions
BANSAL-KINETIC THEORY OF GASES-Solved Example
- 70 calories of heat required to raise the temperature of 2 moles of an...
Text Solution
|
- A vessel contains 1 mole of O(2) and 1 mole of He. The value of gamm...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- Aglass tube scaled at both end is 1 m long. It lies horizontally with ...
Text Solution
|
- Find the amount of work done to increase the temperature of one mole j...
Text Solution
|
- A mercury thermoeter read 80^(@)C when the mercury is at 5.2cm mark an...
Text Solution
|
- A vertical cylinder pistion system has cross-section S. It contains 1 ...
Text Solution
|
- A tyre pumped to a pressure 3.375 atm "at" 27^(@)C suddenly bursts. Wh...
Text Solution
|
- 3 moles of a gas mixture having volume V and temperature T is compress...
Text Solution
|
- One mole of a certain ideal gas is contained under a weight-less pisto...
Text Solution
|
- Calculate the heat absorbed by the system is going through the process...
Text Solution
|
- The mercury manometer consists of two unequal arms of equal cross sect...
Text Solution
|
- An ideal gas has pressure p(0), volumn V(0) and temperature T(0). It i...
Text Solution
|
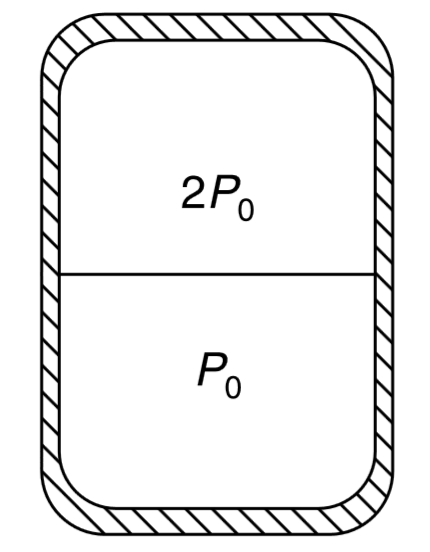
- Show a vertical cylindrical vesse seperated in two parts by a friction...
Text Solution
|
- A cylindrical container of volume V(0) is divided into two parts by a ...
Text Solution
|
- A gas is heated isobarically and the heat used for external work is W....
Text Solution
|
- A vessel contains a mixtrue consisting of m(1) = 7g of nitrogen M(1) =...
Text Solution
|