A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES
BANSAL|Exercise Exercise-2|4 VideosKINETIC THEORY OF GASES
BANSAL|Exercise Reasoning Type|1 VideosKINETIC THEORY OF GASES
BANSAL|Exercise Practice Exercise|3 VideosHEAT TRANSFER
BANSAL|Exercise Exercise|89 VideosUNIT DIMENSION, VECTOR & BASIC MATHS
BANSAL|Exercise Exercise|10 Videos
Similar Questions
Explore conceptually related problems
BANSAL-KINETIC THEORY OF GASES-Exercise 1
- When 2g of gas A is introduced into an evacuated flask kept at 25^(@)C...
Text Solution
|
- The temperature of a gas is doubled (i) on absolute scale (ii) on cent...
Text Solution
|
- The expansion of an ideal gas of mass (m) at a constant pressure (p) i...
Text Solution
|
- One mole of an ideal gas at STP is heated in an insulated closed conta...
Text Solution
|
- A given mass of a gas expands ffrom a state A to the state B by three ...
Text Solution
|
- In the figure shown V(ab) at t=1 is <img src="https://d10lpgp6xz60n...
Text Solution
|
- One mole of an ideal gas is contained with in a cylinder by a friction...
Text Solution
|
- A Polyatomic gas with six degrees of freedom does 25 J of work when it...
Text Solution
|
- A cyclic process ABCA is shown in the V-T diagram process on the P-V
Text Solution
|
- The adiabatic Bulk modulus of a diatomic gas at atmosheric pressure is
Text Solution
|
- A given quantity of a ideal gas is at pressure P and absolute tempera...
Text Solution
|
- Which of the folowing statement is not according to the postulates of ...
Text Solution
|
- N(2) molecules is 14 times heavier than a H(2) molecule. At what tempe...
Text Solution
|
- In a cubical box of volume V, there are N molecules of a gas moving ra...
Text Solution
|
- Two containers are of equal volume.One contains O(2) while the other h...
Text Solution
|
- n molecules of an ideal gas are enclosed in cubical box at temperature...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|
- An ideal gas is taken through series of changes ABCA The amount of wor...
Text Solution
|
- An ideal system can be brought from stage A to B through Four paths as...
Text Solution
|
- In the indicator diagram shown the work done along path AB is:
Text Solution
|
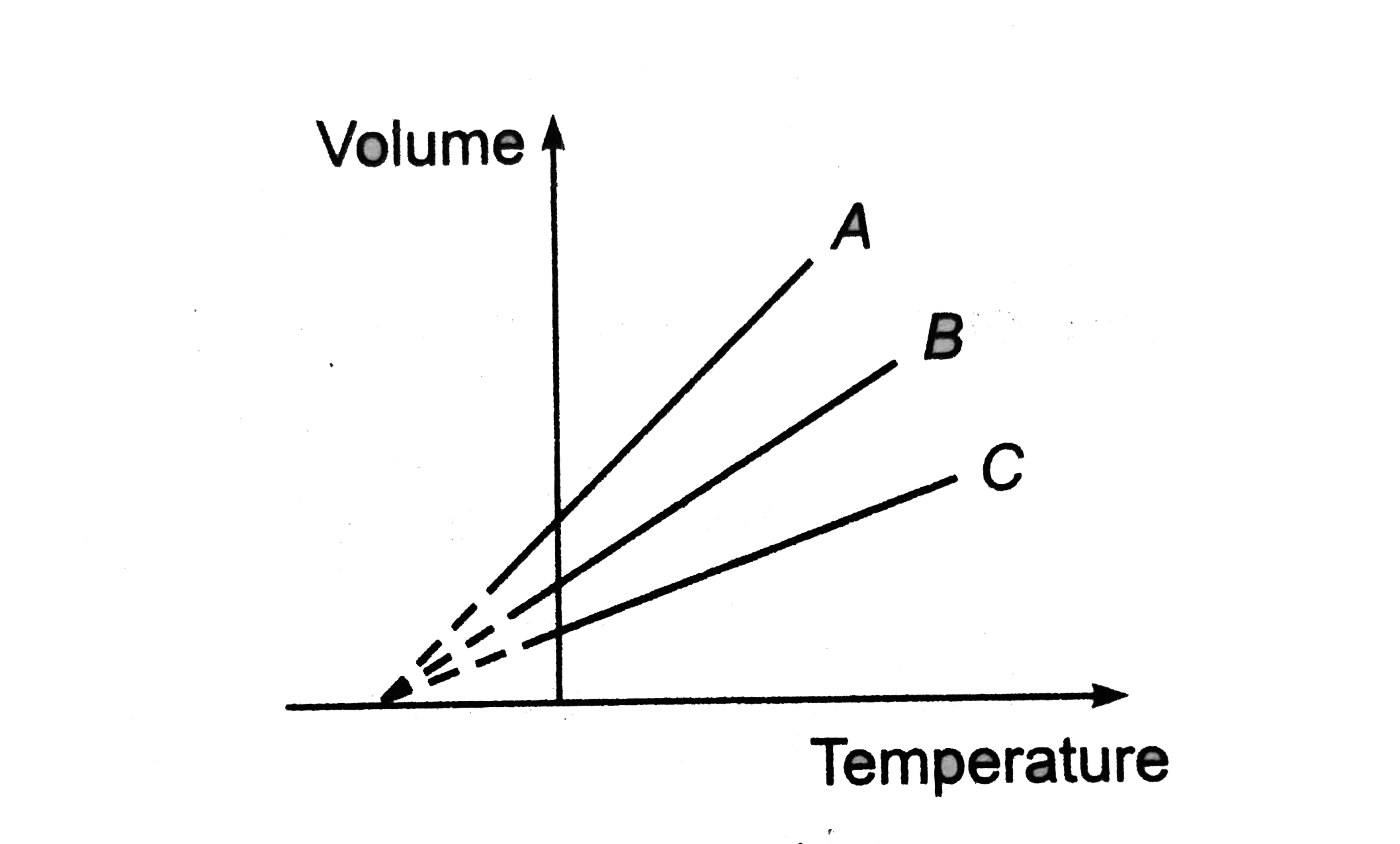 .
.