A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES
BANSAL|Exercise Exercise-2|4 VideosKINETIC THEORY OF GASES
BANSAL|Exercise Reasoning Type|1 VideosKINETIC THEORY OF GASES
BANSAL|Exercise Practice Exercise|3 VideosHEAT TRANSFER
BANSAL|Exercise Exercise|89 VideosUNIT DIMENSION, VECTOR & BASIC MATHS
BANSAL|Exercise Exercise|10 Videos
Similar Questions
Explore conceptually related problems
BANSAL-KINETIC THEORY OF GASES-Exercise 1
- In the indicator diagram shown the work done along path AB is:
Text Solution
|
- In the above problem work done along path BC is:
Text Solution
|
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- During the adiabatic change of ideal gas, the realation between the pr...
Text Solution
|
- A cylindrical tube of uniform cross-sectional area A is fitted with tw...
Text Solution
|
- At a temperature T K, the pressure of 4.0g argon in a bulb is p. The b...
Text Solution
|
- One mole of a monoatomic ideal gas undergoes the process ArarrB in the...
Text Solution
|
- A vessel contains 1 mole of O2 gas (relative molar mass 32) at a tempe...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|
- An idealgas undergoes the process 1 rarr 2 shown in the figure, the he...
Text Solution
|
- The figure shows the graph of logarithmic reading of pressure and volu...
Text Solution
|
- Three processes compose a thermodynamic cycle shown in the accompanyin...
Text Solution
|
- When unit mass of water boils to become steam at 100^(@)C, it absorbs ...
Text Solution
|
- Two identical vessels A & B contain equal amount of ideal monoatomic g...
Text Solution
|
- 2 moles of a monoatomic gas are expanded to double its initial volume,...
Text Solution
|
- An ideal gas is found to obey an additional laq P^(2) V = constant. Th...
Text Solution
|
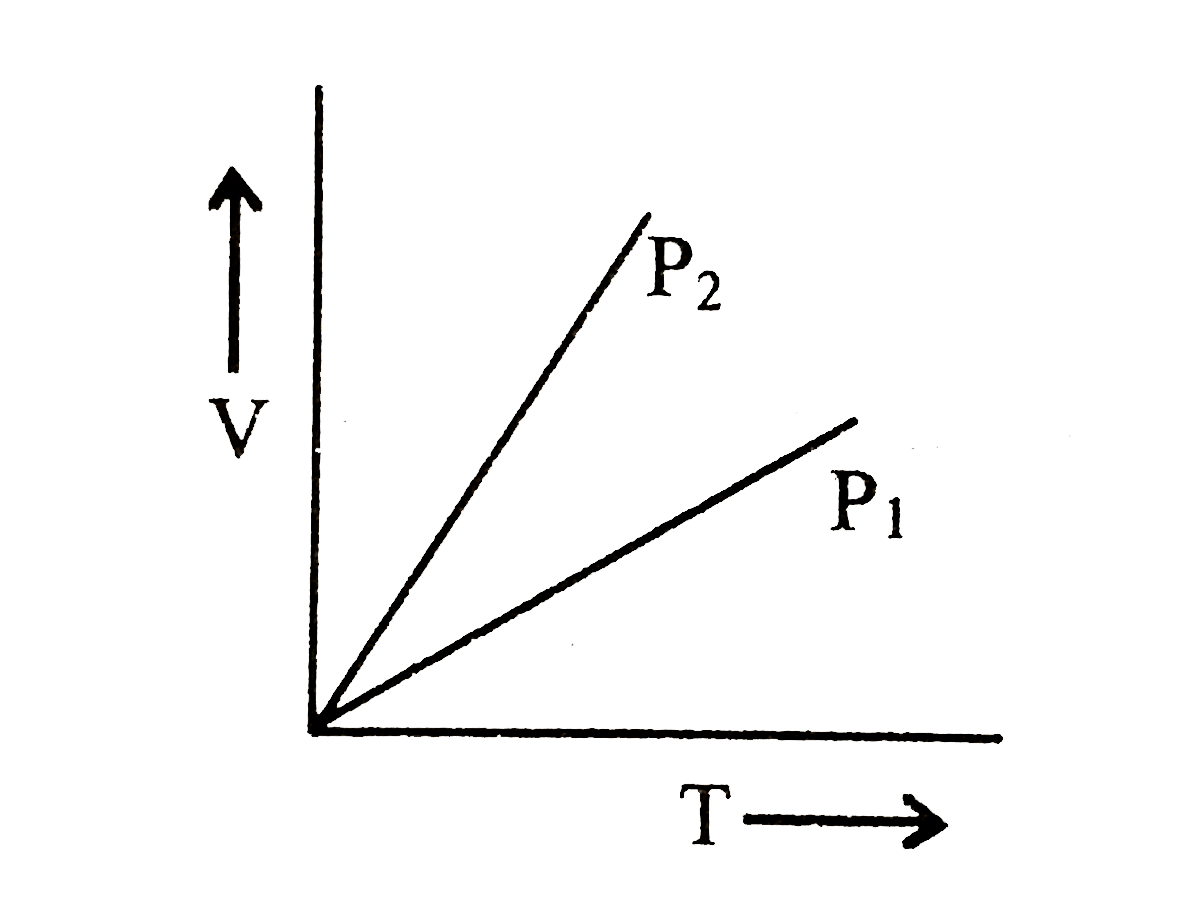
- For V versus T curves at constant pressure P1 and P2 for and ideal gas...
Text Solution
|
- Two different masses m and 3 m of an ideal gas are heated separately i...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|