Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Predict the number of unpaired electrons in a square planar d^(8) ion.
Text Solution
|
- Predict the number of unpaired electrons in square planar [PtCI(4)]^(2...
Text Solution
|
- Predict the number of unpaired electrons in a tetrahedral d^(6) ion an...
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)4...
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)(...
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)4...
Text Solution
|
- Predict the number of unpaired electrons in a square planar d^(8) ion.
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)(...
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)(...
Text Solution
|
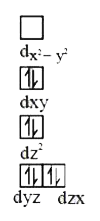 Number of unpaired electron = 1.
Number of unpaired electron = 1.