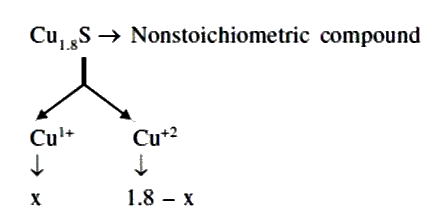Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A certain sample of cuprous sulphide is found to have composition Cu(1...
Text Solution
|
- On hanting a mixture of Cu(2)O and Cu(2)S, we get :
Text Solution
|
- Select the correct statement, for non stoichiometric cuprous oxide Cu(...
Text Solution
|
- A certain sample of cuprous sulphide is found to have composition Cu(1...
Text Solution
|
- A certain sample of cuprous sulphide is found to have the composition ...
Text Solution
|
- When Cu(2)S is converted into Cu^(2+) & SO(2) then equivalent weight o...
Text Solution
|
- A certain sample of cuprous sulphide is found to have composition Cu(1...
Text Solution
|
- Heating a mixture of Cu(2)O and Cu(2)S gives -
Text Solution
|
- Cu(2)O और Cu(2)S के मिश्रण को गर्म करने पर प्राप्त होगा
Text Solution
|